Energy transformation

Energy Transformation in Energy Systems Language.
Energy in many of its forms may be used in natural processes, or to provide some service to society such as heating, refrigeration, lightening or performing mechanical work to operate machines. For example, in order to heat your home, your furnace can burn fuel, whose chemical potential energy is thus converted into thermal energy, which is then transferred to your home's air in order to raise its temperature.
In another example, an internal combustion engine burns gasoline of cause pressure that pushes the pistons, thus performing work in order to accelerate your vehicle, ultimately converting the fuel's chemical energy to your vehicle's additional kinetic energy corresponding to its increase in speed.
Entropy and limitations in conversion of thermal energy to other types
Conversions to thermal energy (thus raising the temperature) from other forms of energy, may occur with essentially 100% efficiency (many types of friction do this). Conversion among non-thermal forms of energy may occur with fairly high efficiency, though there is always some energy dissipated thermally due to friction and similar processes. Sometimes the efficiency is close to 100%, such as when potential energy is converted to kinetic energy as an object falls in vacuum, or when an object orbits nearer or farther from another object, in space.Though, conversion of thermal energy to other forms, thus reducing the temperature of a system, has strict limitations, often keeping its efficiency much less than 100% (even when energy is not allowed to escape from the system). This is because thermal energy has already been partly spread out among many available states of a collection of microscopic particles constituting the system, which can have enormous numbers of possible combinations of momentum and position (these combinations are said to form a phase space). In such circumstances, a measure called entropy, or evening-out of energy distributions, dictates that future states of an isolated system must be of at least equal evenness in energy distribution. In other words, there is no way to concentrate energy without spreading out energy somewhere else.
Thermal energy in equilibrium at a given temperature already represents the maximal evening-out of energy between all possible states. Such energy is sometimes considered "degraded energy," because it is not entirely convertible a "useful" form, i.e. one that can do more than just affect temperature. The second law of thermodynamics is a way of stating that, for this reason, thermal energy in a system may be converted to other kinds of energy with efficiencies approaching 100%, only if the entropy (even-ness or disorder) of the universe is increased by other means, to compensate for the decrease in entropy associated with the disappearance of the thermal energy and its entropy content. Otherwise, only a part of thermal energy may be converted to other kinds of energy (and thus, useful work), since the remainder of the heat must be reserved to be transferred to a thermal reservoir at a lower temperature, in such a way that the increase in Entropy for this process more than compensates for the entropy decrease associated with transformation of the rest of the heat into other types of energy...... Examples
Transformation of kinetic energy of charged particles to electric energy
In order to make the energy transformation more efficient, it is desirable to avoid the thermal conversion. For example, the efficiency of nuclear energy reactors, where kinetic energy of nuclei is first converted to thermal energy and then to electric energy, lies around 35%. By direct conversion of kinetic energy to electric, i.e. by eliminating the thermal energy transformation, the efficiency of energy transformation process can be dramatically improved.Flash Back of energy transformation from the early universe
Energy transformations in the universe over time are (generally) characterized by various kinds of energy which has been available since the Big Bang, later being "released" (that is, transformed to more active types of energy such as kinetic or radiant energy), when a triggering mechanism is available to do it.Release of energy from gravitational potential: A direct transformation of energy occurs when hydrogen produced in the big bang collects into structures such as planets, in a process during which part of the gravitational potential is to be converted directly into heat. In Jupiter, Saturn, and Neptune, for example, such heat from continued collapse of the planets' large gas atmospheres continues to drive most of the planets' weather systems, with atmospheric bands, winds, and powerful storms which are only partly powered by sunlight, however, on Uranus, little of this process occurs.
On Earth a significant portion of heat output from interior of the planet, estimated at a third to half of the total, is caused by slow collapse of planetary materials to a smaller size, with output of gravitationally driven heat.
Release of energy from radioactive potential: Familiar examples of other such processes transforming energy from the Big Bang include nuclear decay, in which energy is released which was originally "stored" in heavy isotopes, such as uranium and thorium. This energy was stored at the time of these elements' nucleosynthesis, a process which ultimately uses the gravitational potential energy released from the gravitational collapse of type IIa supernovae, to store energy in the creation of these heavy elements before they were incorporated into the solar system and the Earth. Such energy locked into uranium is triggered for sudden-release in nuclear fission bombs, and similar stored energies in atomic nuclei are released spontaneously, during most types of radioactive decay. In such processes, heat from decay of these atoms of radioisotope in the core of the Earth is transformed immediately to heat. This heat in turn may lift mountains, via plate tectonics and orogenesis. This slow lifting of terrain thus represents a kind of gravitational potential energy storage of the heat energy. The stored potential energy may be released to active kinetic energy in landslides, after a triggering event. Earthquakes also release stored elastic potential energy in rocks, a kind of mechanical potential energy which has been produced ultimately from the same radioactive heat sources.
Thus, according to present understanding, familiar events such as landslides and earthquakes release energy which has been stored as potential energy in the Earth's gravitational field, or elastic strain (mechanical potential energy) in rocks. Prior to this, the energy represented by these events had been stored in heavy atoms (or in the gravitational potential of the Earth). The energy stored in heat atoms had been stored as potential ever since the time that gravitational potentials transforming energy in the collapse of long-destroyed stars (supernovae) created these atoms, and in doing so, stored the energy within.
Release of energy from hydrogen fusion potential: In other similar chain of transformations beginning at the dawn of the universe, nuclear fusion of hydrogen in the Sun releases another store of potential energy which was created at the time of the Big Bang. At that time, according to theory, space expanded and the universe cooled too rapidly for hydrogen to completely fuse into heavier elements. This resulted in hydrogen representing a store of potential energy which can be released by nuclear fusion. Such a fusion process is triggered by heat and pressure generated from gravitational collapse of hydrogen clouds when they produce stars, and some of the fusion energy is then transformed into sunlight. Such sunlight may again be stored as gravitational potential energy after it strikes the Earth, as (for example) snow-avalanches, or when water evaporates from oceans and is deposited high above sea level (where, after being released at a hydroelectric dam, it can be used to drive turbine/generators to produce electricity). Sunlight also drives many weather phenomena on Earth. An example of a solar-mediated weather event is a hurricane, which occurs when large unstable areas of warm ocean, heated over months, give up some of their thermal energy suddenly to power a few days of violent air movement. Sunlight is also captured by green plants as chemical potential energy, when carbon dioxide and water are converted into a combustible combination of carbohydrates, lipids, and oxygen. Release of this energy as heat and light may be triggered suddenly by a spark, in a forest fire; or it may be available more slowly for animal or human metabolism, when these molecules are ingested, and catabolism is triggered by enzyme action.
Through all of these transformation chains, potential energy stored at the time of the Big Bang is later released by intermediate events, sometimes being stored in a number of ways over time between releases, as more active energy. In all these events, one kind of energy is converted to other types of energy, including heat.
Examples
Examples of sets of energy conversions in machines
For instance, a coal-fired power plant involves these energy transformations:- Chemical energy in the coal converted to thermal energy in the exhaust gases of combustion.
- Thermal energy of the exhaust gases converted into thermal energy of steam through the heat exchanger.
- Thermal energy of steam converted to mechanical energy in the turbine.
- Mechanical energy of the turbine converted to electrical energy by the generator, which is the ultimate output
In a conventional automobile, these energy transformations are involved:
- Chemical energy in the fuel converted to kinetic energy of expanding gas via combustion
- Kinetic energy of expanding gas converted to linear piston movement
- Linear piston movement converted to rotary crankshaft movement
- Rotary crankshaft movement passed into transmission assembly
- Rotary movement passed out of transmission assembly
- Rotary movement passed through differential
- Rotary movement passed out of differential to drive wheels
- Rotary movement of drive wheels converted to linear motion of the vehicle.
Other energy conversions
There are many different machines and transducers that convert one energy form into another. A short list of examples follows:- Thermoelectric (Heat → Electric energy)
- Geothermal power (Heat→ Electric energy)
- Heat engines, such as the internal combustion engine used in cars, or the steam engine (Heat → Mechanical energy)
- Ocean thermal power (Heat → Electric energy)
- Hydroelectric dams (Gravitational potential energy → Electric energy)
- Electric generator (Kinetic energy or Mechanical work → Electric energy)
- Fuel cells (Chemical energy → Electric energy)
- Battery (electricity) (Chemical energy → Electric energy)
- Fire (Chemical energy → Heat and Light)
- Electric lamp (Electric energy → Heat and Light)
- Microphone (Sound → Electric energy)
- Wave power (Mechanical energy → Electric energy)
- Windmills (Wind energy → Electric energy or Mechanical energy)
- Piezoelectrics (Strain → Electric energy)
- Friction (Kinetic energy → Heat)
- Electric heater (Electric energy → Heat)
XXX . XXX 4%zero null 0 1 2 3 4 5 6 Energy quality
Energy quality is the contrast between different forms of energy, the different trophic levels in ecological systems and the propensity of energy to convert from one form to another. The concept refers to the empirical experience of the characteristics, or qualia, of different energy forms as they flow and transform. It appeals to our common perception of the heat value, versatility, and environmental performance of different energy forms and the way a small increment in energy flow can sometimes produce a large transformation effect on both energy physical state and energy. For example the transition from a solid state to liquid may only involve a very small addition of energy. Methods of evaluating energy quality are sometimes concerned with developing a system of ranking energy qualities in hierarchical order.

Since before antiquity there has been deep philosophical, aesthetic and scientific interest in the contrast of quality with quantity. In some respects the history of modern and postmodern thought can be characterized by the phenomenological approach to these two concepts. A central question has been whether the many different qualitative aspects of the world can be understood in terms of rational quantities, or whether the qualitative and quantitative are irreconcilable: that is, there is no "rational quality", or quale ratio. Many scientists and analytic philosophers say they are not, and therefore consider some qualitative phenomena like, for instance, spirituality, and astrology to be unquantifiable, unanalysable by scientific methods, and therefore ungrounded in physical reality. The notion of energy quality therefore has a tendency to be linked with phenomena many scientists consider unquantifiable, or at least incommunicable, and are consequently dismissed out of hand.
At the same time many people have also recognised qualitative differences in the way things can be done by different entities (both physical and biological). Humans, for example have qualitatively different capacities than many other mammals, due, in part, to their opposable thumb. In the attempt to formalise some of the qualitative differences, entities were grouped according to distinguishing features or capacities. Different schools of thought used different methods to make distinctions. Some people chose taxonomic and genome structure, while others chose energetic function as the basis of classifications. The former are often associated with biology, while the latter with the trophic food chain analysis of ecology. These can be considered attempts to formalise quantitative, scientific studies of the qualitative differences between entities. The efforts were not isolated to biology and ecology, since engineers were also interested in quantifying the amount of work that qualitatively different sources of energy could provide.
Receiver methods: view energy quality as a measure and indicator of the relative ease with which energy converts from one form to another. That is, how much energy is received from a transformation or transfer process. For example, A. Grubler used two types of indicators of energetic quality pars pro toto: the hydrogen/carbon (H/C) ratio, and its inverse, the carbon intensity of energy. Grubler used the latter as an indicator of relative environmental quality. However Ohta says that in multistage industrial conversion systems, such as a hydrogen production system using solar energy, the energy quality is not upgraded (1994, p. 125).
Donor methods: view energy quality as a measure of the amount of energy used in an energy transformation, and that goes into sustaining a product or service (H.T.Odum 1975, p. 3). That is how much energy is donated to an energy transformation process. These methods are used in ecological physical chemistry, and ecosystem evaluation. From this view, in contrast with that outlined by Ohta, energy quality is upgraded in the multistage trophic conversions of ecological systems. Here, upgraded energy quality has a greater capacity to feedback and control lower grades of energy quality. Donor methods attempt to understand the usefulness of an energetic process by quantifying the extent to which higher quality energy controls lower quality energy.
At the same time many people have also recognised qualitative differences in the way things can be done by different entities (both physical and biological). Humans, for example have qualitatively different capacities than many other mammals, due, in part, to their opposable thumb. In the attempt to formalise some of the qualitative differences, entities were grouped according to distinguishing features or capacities. Different schools of thought used different methods to make distinctions. Some people chose taxonomic and genome structure, while others chose energetic function as the basis of classifications. The former are often associated with biology, while the latter with the trophic food chain analysis of ecology. These can be considered attempts to formalise quantitative, scientific studies of the qualitative differences between entities. The efforts were not isolated to biology and ecology, since engineers were also interested in quantifying the amount of work that qualitatively different sources of energy could provide.
Ohta
According to Ohta (1994, pp. 90–91) the ranking and scientific analysis of energy quality was first proposed in 1851 by William Thomson under the concept of "availability". This concept was continued in Germany by Z. Rant, who developed it under the title, "die Exergie" (the exergy). It was later continued and standardised in Japan. Exergy analysis now forms a common part of many industrial and ecological energy analyses. For example, I.Dincer and Y.A. Cengel (2001, p. 132) state that energy forms of different qualities are now commonly dealt with in steam power engineering industry. Here the "quality index" is the relation of exergy to the energy content (Ibid.). However energy engineers were aware that the notion of heat quality involved the notion of value – for example A. Thumann wrote, "The essential quality of heat is not the amount but rather its 'value'" (1984, p. 113) – which brings into play the question of teleology and wider, or ecological-scale goal functions. In an ecological context S.E. Jorgensen and G.Bendoricchio say that exergy is used as a goal function in ecological models, and expresses energy "with a built-in measure of quality like energy"Energy quality evaluation methods
There appear to be two main kinds of methodology used for the calculation of energy quality. These can be classed as either receiver or donor methods. One of the main differences that distinguishes these classes is the assumption of whether energy quality can be upgraded in an energy transformation process.Receiver methods: view energy quality as a measure and indicator of the relative ease with which energy converts from one form to another. That is, how much energy is received from a transformation or transfer process. For example, A. Grubler used two types of indicators of energetic quality pars pro toto: the hydrogen/carbon (H/C) ratio, and its inverse, the carbon intensity of energy. Grubler used the latter as an indicator of relative environmental quality. However Ohta says that in multistage industrial conversion systems, such as a hydrogen production system using solar energy, the energy quality is not upgraded (1994, p. 125).
Donor methods: view energy quality as a measure of the amount of energy used in an energy transformation, and that goes into sustaining a product or service (H.T.Odum 1975, p. 3). That is how much energy is donated to an energy transformation process. These methods are used in ecological physical chemistry, and ecosystem evaluation. From this view, in contrast with that outlined by Ohta, energy quality is upgraded in the multistage trophic conversions of ecological systems. Here, upgraded energy quality has a greater capacity to feedback and control lower grades of energy quality. Donor methods attempt to understand the usefulness of an energetic process by quantifying the extent to which higher quality energy controls lower quality energy.
Energy quality in physical-chemical science (direct energy transformations)
Constant energy form but variable energy flow
T.Ohta suggested that the concept of energy quality may be more intuitive if one considers examples where the form of energy remains constant but the amount of energy flowing, or transferred is varied. For instance if we consider only the inertial form of energy, then the energy quality of a moving body is higher when it moves with a greater velocity. If we consider only the heat form of energy, then a higher temperature has higher quality. And if we consider only the light form of energy then light with higher frequency has greater quality (Ohta 1994, p. 90). All these differences in energy quality are therefore easily measured with the appropriate scientific instrument.Variable energy form, but constant energy flow
The situation becomes more complex when the form of energy does not remain constant. In this context Ohta formulated the question of energy quality in terms of the conversion of energy of one form into another, that is the transformation of energy. Here, energy quality is defined by the relative ease with which the energy transforms, from form to form.If energy A is relatively easier to convert to energy B but energy B is relatively harder to convert to energy A, then the quality of energy A is defined as being higher than that of B. The ranking of energy quality is also defined in a similar way. (T.Ohta 1994, p. 90).Nomenclature: Prior to Ohta's definition above, A.W.Culp produced an energy conversion table describing the different conversions from one energy to another. Culp's treatment made use of a subscript to indicate which energy form is being talked about. Therefore, instead of writing "energy A", like Ohta above, Culp referred to "Je", to specify electrical form of energy, where" J" refers to "energy", and the "e"subscript refers to electrical form of energy. Culps notation anticipated Scienceman's (1997) later maxim that all energy should be specified as form energy with the appropriate subscript.
Energy quality in biophysical economics (indirect energy transformations)
The notion of energy quality was also recognized in the economic sciences. In the context of biophysical economics energy quality was measured by the amount of economic output generated per unit of energy input (C.J. Cleveland et al. 2000). The estimation of energy quality in an economic context is also associated with embodied energy methodologies. Another example of the economic relevance of the energy quality concept is given by Brian Fleay. Fleay says that the "Energy Profit Ratio (EPR) is one measure of energy quality and a pivotal index for assessing the economic performance of fuels. Both the direct and indirect energy inputs embodied in goods and services must be included in the denominator." (2006; p. 10) Fley calculates the EPR as the energy output/energy input.
| ||||||||||||||||||||||||||||||||
Ranking energy quality
Energy abundance and relative transformation ease as measure of hierarchical rank and/or hierarchical position
Ohta sought to order energy form conversions according to their quality and introduced a hierarchical scale for ranking energy quality based on the relative ease of energy conversion (see table to right after Ohta, p. 90). It is evident that Ohta did not analyse all forms of energy. For example, water is left out of his evaluation. It is important to note that the ranking of energy quality is not determined solely with reference to the efficiency of the energy conversion. This is to say that the evaluation of "relative ease" of an energy conversion is only partly dependent on transformation efficiency. As Ohta wrote, "the turbine generator and the electric motor have nearly the same efficiency, therefore we cannot say which has the higher quality" (1994, p. 90). Ohta therefore also included, 'abundance in nature' as another criterion for the determination energy quality rank. For example, Ohta said that, "the only electrical energy which exists in natural circumstances is lightning, while many mechanical energies exist." (Ibid.). (See also table 1. in Wall's article for another example ranking of energy quality).Transformity as an energy measure of hierarchical rank
Like Ohta, H.T.Odum also sought to order energy form conversions according to their quality, however his hierarchical scale for ranking was based on extending ecological system food chain concepts to thermodyanmics rather than simply relative ease of transformation . For H.T.Odum energy quality rank is based on the amount of energy of one form required to generate a unit of another energy form. The ratio of one energy form input to a different energy form output was what H.T.Odum and colleagues called transformity: "the EMERGY per unit energy in units of emjoules per joule"XXX . XXX 4%zero null 0 1 2 3 4 5 6 7 8 Simplest Inductive Power Transfer Circuit.
While it is theoretically possible to make a very slightly simpler circuit than that one, this circuit approaches being utterly brilliant in its simplicity. If it works as well as it appears it should then it's an excellent starting point.
The main point to watch is that the transmit and received circuits should be tuned to (or "resonate at") the same frequency. For this to happen the transmit and receive coils need to be the same and the 4.7nF capacitors which are used to "tune" them should also be matched, As 4.7 nF caps may vary by say +/-10% or more depending on the type used, it may be necessary to add a few hundred pF in parallel with one or other of the two 4.7 nF capacitors to get best match
"The capacitors should not be ceramic type". .
Components:
The values shown are standard values and they will be available from most sellers of electronic components.
4.7n = 4.7 nF = 4.7 nano-Farad. Two used.
Voltage is non critical. 10 Volt or more rating is needed but as all 4.7n you will find will be rated at more than 10V you do not need to worry about the voltage rating.
I would have thought ceramic capacitors would be OK, but mylar "plastic" capacitors are common and cheap in 4.7 nF capacity.
470p = 470 pF = 470 pico-Farad.
again, voltage rating is not critical as all will be OK.
Again, ceramic should be OK, but if you find other than ceramic then it would also work. eg maybe mylar or polystyrene.
15k = 15k resistor = 15,000 Ohm resistor.
Any wattage and voltage rating will work.
Any wattage rating OK.
BD139 = BD139 transistor = commonly availableBD139 datasheet

1N4148 = 1N4148 diode = commonly available
LED = Light Emitting diode = almost any LED.
Red & blue circles = transmit and receive coils.
See original for number of turns.
Diameter = 4.5 centimeter.
Connection:
The original video will provide some indication of construction.
Apart from the BD139 and LED all components have only two leads and can be connected in either polarity (or electrical orientation).
If a common 5mm LED is used the longer lead will be the Anode = positive connection. (The NON bar side of the arrow symbol).
BD139 connection is shown above and in the data sheet.
Basic circuit:

Final actual circuit:

In action. Copper tube is used for the inductor to allow high circulating currents with low resistance and so low loss when no power is being transferred.


There are many types of power – physical, social, super, odor blocking, love – but in this tutorial, we’ll be focusing on electric power. So what is electric power?
In general physics terms, power is defined as the rate at which energy is transferred (or transformed).
So, first, what is energy and how is it transferred? It’s hard to state simply, but energy is basically the ability of something to move something else. There are many forms of energy: mechanical, electrical, chemical, electromagnetic, thermal, and many others.
Energy can never be created or destroyed, only transferred to another form. A lot of what we’re doing in electronics is converting different forms of energy to and from electric energy. Lighting LEDs turns electric energy into electromagnetic energy. Spinning motors turns electric energy into mechanical energy. Buzzing buzzers makes sound energy. Powering a circuit off a 9V alkaline battery turn chemical energy into electrical energy. All of these are forms of energy transfers.
| Energy type converted | Converted by |
|---|---|
| Mechanical | Electric Motor |
| Electromagnetic | LED |
| Heat | Resistor |
| Chemical | Battery |
| Wind | Windmill |
Example electric components, which transfer electric energy to another form.
Electric energy in particular, begins as electric potential energy – what we lovingly refer to as voltage. When electrons flow through that potential energy, it turns into electric energy. In most useful circuits, that electric energy transforms into some other form of energy. Electric power is measured by combining both how much electric energy is transferred, and how fast that transfer happens.Producers and Consumers
Each component in a circuit either consumes or produces electric energy. A consumer transforms electric energy into another form. For example, when an LED lights up, electric energy is transformed into electromagnetic. In this case, the lightbulb consumes power. Electric power is produced when energy is transferred to electric from some other form. A battery supplying power to a circuit is an example of a power producer.Wattage
Energy is measured in terms of joules (J). Since power is a measure of energy over a set amount of time, we can measure it in joules per second. The SI unit for joules per second is the watt abbreviated as W.It’s very common to see “watts” preceded by one of the standard SI prefixes: microwatts (µW), miliwatt (mW), kilowatt (kW), megawatt (MW), and gigawatts (GW), are all common depending on the situation.
| Prefix Name | Prefix Abbreviation | Weight |
|---|---|---|
| Nanowatt | nW | 10-9 |
| Microwatt | µW | 10-6 |
| Milliwatt | mW | 10-3 |
| Watt | W | 100 |
| Kilowatt | kW | 103 |
| Megawatt | MW | 106 |
| Gigawatt | GW | 109 |
Microcontrollers, like the Arduino will usually operate in the the µW or mW range. Laptop and desktop computers operate in the standard watt power range. Energy consumption of a house is usually in the kilowatt range. Large stadiums might operate at the megawatt scale. And gigawatts come into play for large-scale power stations and time machines.
Calculating Power
Electric power is the rate at which energy is transferred. It’s measured in terms of joules per second (J/s) – a watt (W). Given the few basic electricity terms we know, how could we calculate power in a circuit? Well, we’ve got a very standard measurement involving potential energy – volts (V) – which are defined in terms of joules per unit of charge (coulomb) (J/C). Current, another of our favorite electricity terms, measures charge flow over time in terms of the ampere (A) – coulombs per second (C/s). Put the two together and what do we get?! Power!To calculate the power of any particular component in a circuit, multiply the voltage drop across it by the current running through it.
Why do we care about the power dropped on a resistor? Or any other component for that matter. Remember that power is the transfer of energy from one type to another. When that electrical energy running from the power source hits the resistor, the energy transforms into heat. Possibly more heat than the resistor can handle. Which leads us to…power ratings.
Power Ratings
All electronic components transfer energy from one type to another. Some energy transfers are desired: LEDs emitting light, motors spinning, batteries charging. Other energy transfers are undesirable, but also unavoidable. These unwanted energy transfers are power losses, which usually show up in the form of heat. Too much power loss – too much heat on a component – can become very undesirable.Even when energy transfers are the main goal of a component, there’ll still be losses to other forms of energy. LEDs and motors, for example, will still produce heat as a byproduct of their other energy transfers.
Most components have a rating for maximum power they can dissipate, and it’s important to keep them operating under that value. This’ll help you avoid what we lovingly refer to as “letting the magic smoke out”.
Resistor Power Ratings
Resistors are some of the more notorious culprits of power loss. When you drop some voltage across a resistor, you’re also going to induce current flow across it. More voltage, means more current, means more power.Remember back to our first power-calculation example, where we found that if 9V were dropped across a 10Ω resistor, that resistor would dissipate 8.1W. 8.1 is a lot of watts for most resistors. Most resistors are rated for anywhere from ⅛W (0.125W) to ½W (0.5W). If you drop 8W across a standard ½W resistor, ready a fire extinguisher.
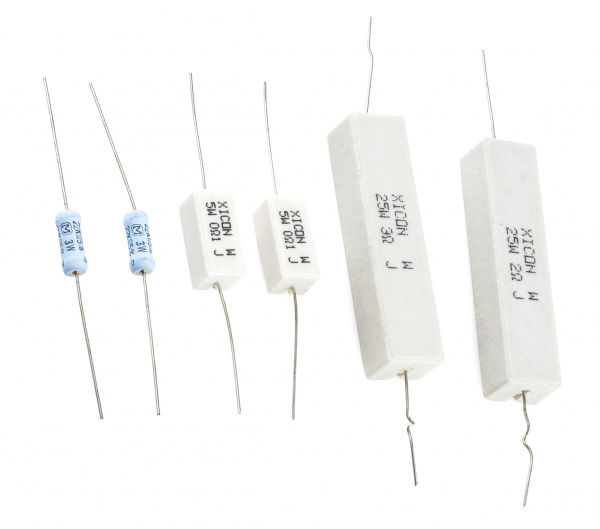
If you’ve seen resistors before, you’ve probably seen these. Top is a ½W resistor and below that a ¼W. These aren’t built to dissipate very much power.
There are resistors built to handle large power drops. These are specifically called out as power resistors.
These large resistors are built to dissipate lots of power. From left to right: two 3W 22kΩ resistors, two 5W 0.1Ω resistors, and 25W 3Ω and 2Ω resistors.
If you ever find yourself picking out a resistor value. Keep it’s power rating in mind as well. And, unless your goal is to heat something up (heating elements are basically really high-power resistors), try to minimize power loss in a resistor.For Example
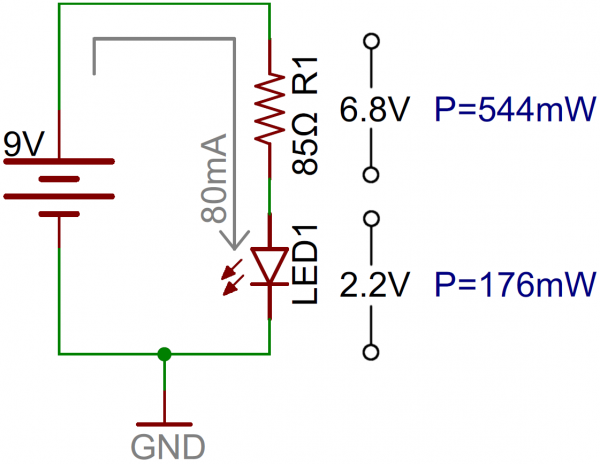
Resistor power ratings can come into play when you’re trying to decide on a value for an LED current-limiting resistor. Say, for example, you want to light up a 10mm super-bright red LED at maximum brightness, using a 9V battery.That LED has a maximum forward current of 80mA, and a forward voltage of about 2.2V. So to deliver 80mA to the LED, you’d need an 85Ω resistor to do so.
6.8V dropped on the resistor, and 80mA running through it means 0.544W (6.8V*0.08A) of power lost on it. A half-watt resistor isn’t going to like that very much! It probably won’t melt, but it’ll get hot. Play it safe and move up to a 1W resistor (or save power and use a dedicated LED driver).
Resistors certainly aren’t the only components where maximum power ratings must be considered. Any component with a resistive property to it is going to produce thermal power losses. Working with components that are commonly subjected to high power – voltage regulators, diodes, amplifiers, and motor drivers, for example – means paying extra special attention to power loss and thermal stress.
Energy transfer diagrams may be used to show the locations of energy stores and energy transfers. For example, consider the energy transfers in this simple electrical circuit:
We can show the transfers like this:
The battery is a store of internal energy (shown as chemical energy). The energy is transferred through the wires to the lamp, which then transfers the energy to the surroundings as light. These are the useful energy transfers - we use electric lamps to light up our rooms.
But there are also energy transfers that are not useful to us. In the example above, the lamp also transfers energy to surroundings by radiation as infrared light, which increases the temperature of the surroundings. If we include this energy transfer (shown as thermal energy in the diagram), the diagram looks like this:
Energy stores and transfers
Energy can be stored and transferred. It cannot be created or destroyed. The total energy of a system stays the same. Energy is transferred from a hot object to a cool one by conduction or radiation
Conservation of energy
In science, a system is a set of things and the processes that happen in them and between them. Energy can be stored or transferred, but it cannot be created or destroyed. This means that the total energy of a system stays the same. The idea that the total energy has the same value before and after a change is called conservation of energy.
For example, an electric lamp might transfer radiant energy to the surroundings. Some of this will be visible light and some will be infrared red light, which increases the temperature of the surroundings. The amount of energy transferred from the lamp will be the same as the amount of energy transferred into the lamp because energy can never be created or destroyed.
Sankey diagrams
You can show energy transfers in a Sankey diagram. In these diagrams, the thicker the line or arrow, the greater the amount of energy involved.
Notice that 100 J of electrical energy is transferred to the lamp. Of this, 10 J is transferred to the surroundings as light energy. The remainder, 90 J (100 J – 10 J) is transferred to the surroundings as radiant energy that increases the temperature of the surroundings. Energy can never be created or destroyed so the energy at the beginning (here 100 J) must equal the total of the energy at the end (here also 100 J).
The energy transfer as light to the surroundings is the useful transfer. The rest is ‘wasted’ - it just makes the surroundings warmer rather than helping you see where you are going. This ‘wasted’ energy eventually becomes so spread out that it becomes useless.
Heating and temperature
Temperature
A thermometer is used to measure the temperature of an object
The temperature of an object is to do with how hot or cold it is, measured in degrees Celsius. Note that the unit of temperature is written as °C, (not °c or oC).
All objects contain internal energy. Some of this is due to the movement of the particles in the object. When an object is heated, its particles move more vigorously and its internal energy increases. Unless the object changes state (eg melts or boils), its temperature will increase.
Example 1
A swimming pool at 30°C is at a lower temperature than a cup of tea at 80°C. But the swimming pool contains more water, so it stores more internal energy than the cup of tea.
Example 2
To boil water we must increase its temperature to 100°C. It takes longer to boil a large beaker of water than a small beaker. This is because the large beaker contains more water and needs to gain more internal energy to reach 100°C.
Thermal equilibrium
If there is a difference in temperature between two objects, energy is transferred from the hotter object to the cooler one. This will continue until both objects are at the same temperature. When they are at the same temperature, we say that they are in thermal equilibrium, and there is no overall transfer of energy any more between the two objects.
Energy can be transferred from a hot object to a cooler one by:
- conduction (if they are touching each other)
- convection
- radiation
Conduction
When a substance is heated, its particles gain internal energy and move more vigorously. The particles bump into nearby particles and make them vibrate more. This passes internal energy through the substance by conduction, from the hot end to the cold end.
This is how the handle of a metal spoon soon gets hot when the spoon is put into a hot drink.
- A substance that transfers energy easily from the hot part to the cold part is called a conductor. Metals are good conductors.
- A substance that does not transfer energy easily from the hot part to the cold part is called an insulator. Air and plastics are insulators.
Convection
The particles in liquids and gases can move from place to place. Convection happens when particles with a lot of thermal energy in a liquid or gas move, and take the place of particles with less thermal energy. Thermal energy is transferred from hot places to cold places by convection.
Radiation
All objects transfer energy to their surroundings by infrared radiation. The hotter an object is, the more infrared radiation it gives off.
No particles are involved in radiation, unlike conduction. This means that energy transfer by radiation can work when objects are not touching, even in space:
- radiation is why we are warmed by the Sun, even though it is millions of kilometres away in space
- infrared cameras give images even in the dark, because they are detecting infrared light, not visible light

In this thermogram of a pan on a stove, the hottest parts are coloured white, yellow or red; the coldest parts are coloured purple or black
The differences between conduction, convection and radiation
Insulation
Take a look at this thermogram of a house. Its roof and windows are the hottest, showing that most energy is lost from the house that way.

A thermogram of house, showing areas of heat loss
Energy is transferred from warm homes to the outside by:
- conduction through the walls, floor, roof and windows
- radiation from the walls, roof and windows
Ways to reduce energy transfer
There are some simple ways to reduce energy transfers from a house, including fitting:
- carpets and curtains
- reflective foil on the inside walls
- double glazing
Double glazing involves having two panes of glass in the window instead of just one. There is air or an even better insulator such as argon gas between the two panes of glass. This reduces energy transfer by conduction.
Energy loss through walls can be reduced using cavity wall insulation. This involves blowing insulating material into the gap between the outside wall and the inside wall to reduce conduction. Loft insulation works in a similar way.
How people and animals use insulation to keep warm
that transformity is a dimensionless ratio.
Definition as a ratio
One part of the rationalist viewpoint associated with modernity and science is to contrast qualitatively different phenomena under transformation through quantitative ratios, with the aim of uncovering any constancy amidst the transformation change. Like the efficiency ratio, transformity is quantitatively defined by a simple input-output ratio. However the transformity ratio is the inverse of efficiency and involves both indirect and direct energy flows rather than simply direct input-output energy ratio of energy efficiency. This is to say that it is defined as the ratio of emergy input to energy output.Original version::
Development
However, it was realised that the term "energy output" refers to both the useful energy output and the non-useful energy output. (Note: that as given by P.K.Nag, an alternative name for 'useful energy' is 'availability' or exergy, and an alternative name for 'non-useful energy' is 'unavailability', or anergy (Nag 1984, p. 156)). But as E.Sciubba and S.Ulgiati observed, the notion of transformity meant to capture the emergy invested per unit product, or useful output. The concept of Transformity was therefore further specified as the ratio of "input emergy dissipated (availability used up)" to the "unit output exergy" (Sciubba and Ulgiati 2005, p. 1957). For Jørgensen (2000, p. 61) transformity is a strong indicator of the efficiency of the system.Revised version: or
Substituting in the mathematical definition of emergy given in
XXX . XXX 4%zero null 0 1 2 3 4 5 6 7 8 Wireless Energy Transfer May Power Devices at a Distance
scientists are perfecting a new method for transmitting electrical energy from a base station using a technique that resembles a wireless Internet connection. Researchers say that a specially designed device should be able to draw power from a strong magnetic field permeating a room.
The effect, which has not yet been demonstrated, would take advantage of the stationary magnetic field that surrounds a charged loop of metal. This so-called near-field can be powerful--it is what makes an electric motor turn. And in principle its oscillations can induce an electric current in another nearby loop, because dynamic magnetic fields create electric fields and vice versa. The second loop could act as a battery or recharger, but it would normally receive only a slight current because the near field fades rapidly over distance. The key to boosting the induced current is resonance, says photonics . When electrified, such an object has a natural, or resonant, frequency that results from current flowing back and forth along the loop from one disk to the other. If two loops have the same frequency, one should be able to receive energy from the other through the magnetic near field--much like two identical tuning forks brought into close proximity, the group reasoned. From a few meters away, the rate of energy transferred in this way might reach tens of watts, or enough to power a laptop .