processing the snow in the sky

Snowflakes are born inside clouds at high
elevations, where temperatures plunge to well below the freezing point
of water. Silicate materials – clay minerals and micas – can act as the
core of a fledgling snow crystal.
Water molecules join together in a rigid
pattern – a tiny ice structure that’s the heart of a snow crystal. The
crystal grows by continuing to collect water vapor, and by the
attachment of water droplets to its surface.
As it grows, it gets heavier. It starts
falling through the cloud. On its way down, it encounters millions more
drops of water. The ice crystal gets bigger as the droplets in the cloud
give their water to it. All over the cloud, the number of ice crystals
multiplies – and soon the whole cloud might be ice. If the flakes of ice
get heavy enough, they might fall out of the cloud, drift down to
Earth, and blanket the ground as snow.

Schematic diagram of the conceptual model for snow formation from arctic clouds such
How does snow form?

Snow is formed when temperatures are low and there is moisture in the atmosphere in the form of tiny ice crystals.
When these tiny ice crystals collide they stick together in clouds to become snowflakes.If enough ice crystals stick together, they'll become heavy enough to fall to the ground.
this:
How does snow form?

Snow is formed when temperatures are low and there is moisture in the atmosphere in the form of tiny ice crystals.
When these tiny ice crystals collide they stick together in clouds to become snowflakes.If enough ice crystals stick together, they'll become heavy enough to fall to the ground.
How cold does it have to be to snow?
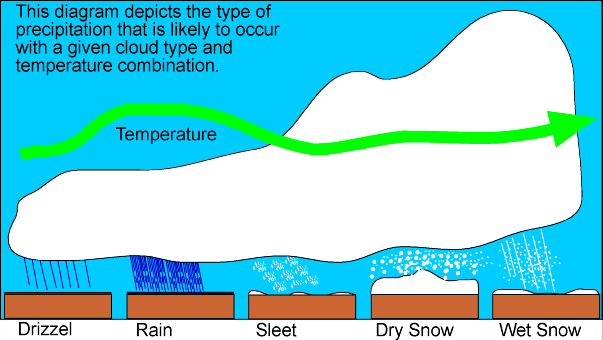
Precipitation falls as snow when the air temperature is below 2 °C. It is a myth that it needs to be below zero to snow. In fact, in this country, the heaviest snow falls tend to occur when the air temperature is between zero and 2 °C. The falling snow does begin to melt as soon as the temperature rises above freezing, but as the melting process begins, the air around the snowflake is cooled.If the temperature is warmer than 2 °C then the snowflake will melt and fall as sleet rather than snow, and if it's warmer still, it will be rain.
'Wet' snow vs. 'dry' snow
The size and make up of a snowflake depends on how many ice crystals group together and this will be determined by air temperatures. Snowflakes that fall through dry, cool air will be small, powdery snowflakes that don't stick together. This 'dry' snow is ideal for snow sports but is more likely to drift in windy weather.When the temperature is slightly warmer than 0 °C, the snowflakes will melt around the edges and stick together to become big, heavy flakes. This creates 'wet' snow which sticks together easily and is good for making snow men.
Snowflakes
Snowflakes are collections of ice crystals that can occur in an infinite variety of shapes and forms - including prisms, hexagonal plates or stars. Every snowflake is unique, but because they join together in a hexagonal structure they always have six sides.At very low temperatures snowflakes are small and their structure is simple. At higher temperatures the individual flakes may be composed of a very large number of ice crystals - making a complex star shape - and can have a diameter of several inches.
Snowflake

Find out more about snowflakes. How do they form and what gives them their unique appearance?
Snowflakes are one of the most recognisable symbols of winter
weather. Every snowflake is unique and there is an infinite number of
possible shapes they can form.What is a snowflake?
A snowflake begins life as a tiny droplet of supercooled water which freezes in the sky to create an ice crystal. The droplet becomes frozen either because temperatures are sufficiently cold (−35 °C or lower) to freeze to other droplets, or in warmer clouds, they can form around a nucleus such as a dust or pollen particle.Once the ice crystal has formed, if the conditions are right it will begin to grow as water molecules in the air are deposited onto the ice crystal which aggregate as the ice crystal falls to the ground to form a snowflake.
Snowflakes have six sides
All snowflakes contain six sides or points owing to the way in which they form. The molecules in ice crystals join to one another in a hexagonal structure, an arrangement which allows water molecules - each with one oxygen and two hydrogen atoms - to form together in the most efficient way. It is these hexagonal structures which when repeated successively give a snowflake it six-sided shape.Snowflakes are unique
Part of the enduring appeal of snowflakes is their intricate appearance and near infinite variation, often leading to the assertion that all snowflakes are unique. While this in some sense impossible to test, as a general rule it is entirely true. The number of possibilities of nuances in temperature and humidity as the snowflake falls to the ground is limitless. If you look closely at a snowflake you will see countless individual features, all of which could have formed ever so slightly differently in direction or shape owing to the slightest change in the environment in which it formed.What makes snowflakes white?
While snowflakes appear white as they fall through the sky, or as they accumulate on the ground as snowfall, they are in fact totally clear. The ice though is not transparent like a sheet of glass is, but rather is translucent meaning light passes through but not directly. The many sides of the ice crystals cause diffuse reflection of the whole light spectrum which results in snowflakes appearing to be white in colour.Types of snowflake
Whilst the variation of snowflake shapes is infinite, they can be broadly categorised into groups which give an indication as to what conditions the snowflake formed in. Three of the broad types are:Dendrite snowflake
Perhaps the most eye-catching type of snowflake, the name means 'tree-like' recognising their branching structures . They form in very low temperatures of -20 to -25 °C where the atmosphere contains abundant moisture to allow the snowflake to form intricate patterns.
 Thin plate
Thin plate
Plates are essentially part-formed dendrites, the begin to form their
intricate patterns but cannot obtain sufficient moisture to form the
branches so form a less intricate flat plate. These tend to form at
warmer temperatures where there is less moisture in the air.Sector plate
Similar to a thin plate, a sector plate snowflake again lacks moisture but forms a hexagonal structure often with a star-like shape in the centre and the more visible attempts to branch.

Snowflakes
Item Water Cooler than Ice?Most people recognize the analogy of snow as rain when the air temperature is cold enough. Therefore,
the general conditions necessary for the descent of a rain in general
can be used as a condition for snowfall in a region, in addition to the
criteria in the form of a temperature close to 0 ~ ^ \ circ {\ rm C}. The
composition of standard large collection of rain itself in the form of
water vapor and style that lifts higher water vapor into the atmosphere.
This lifting force to bring moisture to the cooler parts of the atmosphere so that water vapor condenses into drops of water. This collection of water droplets then appear as a cloud.In case of rain, the story goes is quite simple. The drops will merge with one another. One moment the drops until a condition is too severe to survive in the cloud, affected gravity to plunge to Earth. However, in the case of snow, the story is slightly different.Might easily imagine, the temperature is cold enough, the drops can also be frozen into ice crystals. Cold as what? Zero degrees Celsius? Apparently not! Let unloading: beads of the new pure water is absolutely freezing when the temperature had dropped to -48 ~ ^ \ circ {\ rm C}. Starting at that temperature, the water droplets will freeze automatically.
But during this time the water freezes at a temperature of 0 ~ ^ \ circ {\ rm C}, instead of -48 ~ ^ \ circ {\ rm C}? True, the temperature of 0 ~ ^ \ circ {\ rm C} is the temperature of melting ice, and liquid water freezes. The proof, the Celsius thermometer, the scale is based on the freezing and boiling water, noting the freezing point of water at zero degrees.
Then, how water droplets can survive in the liquid state to temperatures as cold as it was?
A decisive difference in this case is related to the purity of water droplets. At the point of almost pure water, everywhere there is only water molecules that are uniform and freezing. Because there is almost no difference in the water big group, these water molecules feel comfortable with the situation, and not interested in a unified build ice crystals.

Nucleation process, in the form of carbon dioxide bubbles attached to the ring
So that the water molecules coalesce soon build ice crystals without cooling to -48 ~ ^ \ circ {\ rm C}, it is necessary "interference" from outside. "Nuisance" the most common in this case is to provide speck-related variability from water droplets, for example by adding a microscopic dust grain. The addition of a microscopic speck of dust will disturb 'comfort' molecule that resides in the water droplets, and push the water molecules to join. Molecules that joined the microscopic dust is not like jostling so that they will set up their positions in an orderly and neat, undergo a process called "nucleation", which literally means "nucleation". As long as the ambient temperature is below 0 ~ ^ \ circ {\ rm C}, nucleation will continue such that the alloy, structured water molecules that form an ice crystal.(Xsample : nucleation process also plays a role in the formation of foam in the bottom of the pot of boiling water, or in a bottle of soda, etc.)Freezing Rain, Snow Is NotNow, suppose the cloud has accommodated many ice crystals continue to grow, as is true for rain water droplets, ice crystals continue to grow up too heavy to still inhabit the cloud. So, gang-insured the ice crystals fall to the Earth, while 'recruit' drops of water that may be encountered.Of course, the Earth's atmosphere is not a single entity uniform air masses. The water content in the parts of the atmosphere can be different than in other parts of the water content. Temperatures in the layers of the atmosphere was also different from the temperature in the other layers. These differences, mainly related to temperature, produces 4 types of precipitation (the result of condensation of water vapor in the atmosphere falls back to Earth) in the winter.

The types of precipitation in winter. Orange area represents the mass of warm air (temperature above zero degrees Celsius), while blue areas represent the mass of cold air (temperatures below zero degrees Celsius).
These four types of precipitation are distinguished by the presence of a warm air mass (above 0 ~ ^ \ circ {\ rm C}) on its way to Earth:
?? In the first condition, warm air masses dominate the path of the ice crystals, including near the ground, so that the ice crystals melt immediately and remains in a liquid state when she touched the face of the Earth. Which is known as a type of rain.
The second condition, instead accompanied by a warm air mass along the way, crystals of ice that had melted it again met the cold air mass. Beads of water does not have time to freeze before reaching the entire face of the Earth, but freezes on contact with the objects on the surface, overlaying it with ice. The kind that is known as 'freezing rain' (freezing rain).
The third condition, warm air masses were encountered ice crystal grains are not as thick as in the first and second conditions. Therefore, crystal grains of ice that has melted it can refreeze, forming tiny beads of ice. This type is known as 'sleet', which could be interpreted as "hail" according to Wikipedia in the Malay language.
The fourth condition, there is no significant mass of warm air to melt the ice crystals so that these crystals can reach the Earth's surface remains in crystalline form. This form is commonly called snow.Especially for the third condition, perhaps some of us are going to compare it to 'hail' which sometimes occur in some parts of Indonesia, or the English term 'hail'. However, different from sleet hail by large ice particles are dropped. Sleet tend to be smaller, about the size of a pinhead, while hail can sometimes reach the size of marbles or more.Additionally, sleet can occur in all the clouds that bring enough water reserves, while hail forms under storm clouds, known as cumulonimbus. Chip size fairly large hail are the result of the swirling movement between the bottom and top of the clouds, the temperature enough to freeze the drops there.

Cumulonimbus, besides the danger of hail (hail), the cloud is also synonymous with the condition of thunderstorms and strong winds.
In accordance with its characteristics, sleet occurs only when the air temperature near the ground is below freezing. On the other hand, hail can still occur when the air temperature is well above freezing (above 15 ~ ^ \ circ {\ rm C}) because the pieces of ice that formed large enough so the opportunity is not exhausted melt before it gets to Earth ,Snow quirky piecesPreviously had discussed that differences in atmospheric conditions in the course of an ice crystal can produce a certain difference. The differences that emerged, in turn, helped shape the flakes falling with each shape is unique.On his way fell to Earth, the snowflakes always 'recruit' drops of water that he met on his journey. How and where these points combine snow crystals expand will depend on the environmental conditions when one by one drops of water to join her.Research and taste for police people will form snowflakes have made little headway when Wilson A. Bentley (1865-1931) published a photography, which utilize a microscope to shoot up to 5,000 pieces of snow. Results Bentley photo shows the vast diversity between forms snowflakes were photographed, although generally forms such diverse snowflakes were then grouped in 1951. IACS (International Association of Cyrospheric Sciences) is an organization which groups forms snowflakes into 10 common forms.
 \
\The classification of 10 common forms snowflakes
Various studies further by Kenneth Libbrecht, a professor of physics at the California Institute of Technology, drill down further the relationship between air conditions (especially humidity and temperature) with the resulting shape of snowflakes. In general, the dry and cold air conditions during the formation, the more simple form of snowflakes are formed.

the relationship between humidity, temperature, and the shape of snowflakes are formed.
See sensitivity in this process, it can be concluded that in practice it is very difficult to get two snowflakes are identical. If the eccentric ... maybe a lot ... But, of the many pieces of the snow, or ten approved form groupings, all this snowflake crystals adhere to the same layout, ie hexagons. The hexagonal shape is derived from the composition of constituent hydrogen and oxygen atoms of water molecules, yangseluruhnya wake arranged in hexagons, eventually becoming the unity of snowflakes that is also based on the hexagon shape.
Thus, if we are given a system image with crystal snowflakes pentagonal, or seven, most likely it's not a piece of real snow. Despite the occasional snowflake angled three or twelve could be formed.
What makes clouds, rain, snow, hail and sleet
When warm, wet air rises, it cools, and water vapor condenses out to form clouds. A cloud is made up of small drops of water or ice crystals, depending on its height and how cold is the surrounding air. Height and temperature also determine whether any "precipitation" which results (from the Latin for "to fall from") will be rain or the hail associated with thunderstorms, or the snow, sleet and freezing rain we associate with winter weather.
 To
form rain, water vapor needs what's called a "condensation nucleus", which
can be tiny particles of dust, or pollen, swept up high into the atmosphere.
When the condensing droplets that form the cloud get large and heavy enough
to overcome the upward pressure of convection, they begin to fall. If the
temperature all the way to the ground is above freezing, then--it's raining!
When ice crystals form high up in the cloud, and it's below the freezing point
of water all the way down, then you get snow. But when there are alternating
layers of air above and below freezing, you get other types of precipitation.
To
form rain, water vapor needs what's called a "condensation nucleus", which
can be tiny particles of dust, or pollen, swept up high into the atmosphere.
When the condensing droplets that form the cloud get large and heavy enough
to overcome the upward pressure of convection, they begin to fall. If the
temperature all the way to the ground is above freezing, then--it's raining!
When ice crystals form high up in the cloud, and it's below the freezing point
of water all the way down, then you get snow. But when there are alternating
layers of air above and below freezing, you get other types of precipitation.
 If
a snowflake falls through a region of the cloud where there's liquid water
which coats the flake with more and more layers of new ice, you begin to get
hail. When the thunderstorm's updrafts are strong enough, some of the young
hailstones are swept back up and repeat their journey, getting coated with
more and more layers of ice. Eventually they grow so big that not even the
strongest updraft can keep them aloft, and so they fall to Earth, in sizes
from that of a pea to a golf-ball, and up to the record holder--6 inches long
and 17 inches in circumference! (Kansas, 1970.)
If
a snowflake falls through a region of the cloud where there's liquid water
which coats the flake with more and more layers of new ice, you begin to get
hail. When the thunderstorm's updrafts are strong enough, some of the young
hailstones are swept back up and repeat their journey, getting coated with
more and more layers of ice. Eventually they grow so big that not even the
strongest updraft can keep them aloft, and so they fall to Earth, in sizes
from that of a pea to a golf-ball, and up to the record holder--6 inches long
and 17 inches in circumference! (Kansas, 1970.)
As
damaging as hail can be to houses and especially to agriculture, freezing
rain can be even more lethal, especially to travelers, as they bring "ice
storms" like those of 1998. Freezing rain occurs when earth and objects on
the surface, such as roads, tree limbs and power cables, are at temperatures
below 0° Celsius, 32° F. Above the ground, however, falling snow first
encounters a layer of somewhat warmer air, which melts the flakes, and then,
right above the surface, a very cold layer, which makes the liquid water "super-cooled",
ready to freeze up at the slightest provocation. The trigger is encountering
the freezing surfaces: what results is a thin, sometimes transparent film
of ice.
 The
weight of the ice can cause tree limbs to fall across power lines, or sometimes
just drag down the lines themselves. Driving conditions are, of course, very
dangerous. This is one of the factors that make extreme heat and extreme cold
the #1 weather killers in the United States--even more lethal than lightning,
floods, tornadoes and hurricanes.
The
weight of the ice can cause tree limbs to fall across power lines, or sometimes
just drag down the lines themselves. Driving conditions are, of course, very
dangerous. This is one of the factors that make extreme heat and extreme cold
the #1 weather killers in the United States--even more lethal than lightning,
floods, tornadoes and hurricanes. X . I
How does the process of falling snow?

To answer that, we can start the process of snow. Starting from the water vapor in Earth's atmosphere are assembled, a collection of water vapor cools to the point of condensation (ie the temperature at which the gas is transformed into liquid or solid), then coagulates to form clouds. At the beginning of the formation of clouds, its mass is much smaller than the mass of air that the clouds float in the air just like the wooden beams that floats on the water surface. However, once the vapor collection continues to grow and merge into the clouds, its mass also increases, so that at some point the air can no longer hold it. The clouds broke and the water particles fell to Earth.Particles falling water is pure water (not contaminated by other particles). Pure water is not immediately freezes at 0 degrees Celsius, because at that temperature changes phase from liquid to solid. To make pure water freezing temperature required is lower than 0 degrees Celsius. This also happens when we boil water, the water evaporates when the temperature is above 100 degrees Celsius for at 100 degrees Celsius is a phase change from liquid to vapor. To accelerate the phase change of a substance, usually added special substances, such as salt used to accelerate the melting of the ice into the water phase.Usually the air temperature just below the cloud is below 0 degrees Celsius (air temperature depending on height above sea level). However, low temperature alone is not enough to create snow. When particles of pure water is in contact with air, pure water are contaminated by other particles. There are certain particles that function accelerates the freezing phase, so that the pure water quickly become ice crystals.Impurity particles involved in this process as nukleator, in addition to functioning as an accelerating phase of freezing, also adhesive water antaruap. So the water particles (which is not pure anymore) join together with other water particles to form larger crystals.If the air temperature is not to melt the ice crystals, ice crystals fall to the ground. And this is the snow! If not, the ice crystals melt and get to the ground in the form of rain water.In many cases in the world, the rain always starts with some snow when he fell from the clouds, but then melts as it crosses the warm air. Sometimes, if a very low temperature, ice crystals can form small balls of ice and there was hail. Bandung including relatively frequent hail. So, this is why the snow is very hard down naturally in tropical regions that have relatively high air temperature over the region who are experiencing winter.Possesses a unique structure, there is no snow crystals that have the same shape in this world as our fingerprints. Imagine, snow has fallen since the earth is created until now, and none of snow that have the same crystal structure forms!The uniqueness of the snow the other is the white color. If the snow is heavy, the expanse of the earth to white, clean, and seemed to glow. This is due to the structure of snow crystals allow snow to reflect all the colors in all directions in the same amount, then comes the white color. The same phenomenon can we find at the sight of white sand, chunks of salt, sugar lumps, fog, clouds, and white paint.In addition, the snow gives warmth. It can be understood from the concept of effective temperature. The effective temperature is the temperature perceived by our skin, is influenced by three physical quantities: measured temperature (thermometer), the speed of air movement, and humidity. The effective temperature is typically used to define a "comfort zone". On the beach, the measured temperature can be high, but due to the strong winds we still feel comfortable. At the time of heavy snow, humidity rises and this affects the effective temperature so that on the one condition we feel warm.
X . II
snow fact
Snow fact actually made of water vapor in the Earth's atmosphere gathered. This set of water vapor cools to the temperature at which gas can be changed into a liquid or solid that is called condensation. After that, the results of earlier clot forming cloud condensation. Same process as rain, the amount of water vapor to form clouds, then increasingly also weighed. And when the air is already guns could no longer hold it, the cloud will burst and the clot had issued a water particles.Water particles that fall to Earth is pure water lhoo Friends Orbit, this means the water is not mixed with any substance that can pollute. As we learned in science lessons, the new pure water may freeze when the air temperature is lower than 0 degrees Celsius and the air temperature is usually just below the clouds is below 0 degrees Celsius, but this also depends on how high above sea level. Well, when particles of pure water falls and contact with air, pure water was immediately turned into ice crystals. But it was not just the air that makes the water into ice, a touch of dirt and other particles in the air or referred to Nukleator process that makes the process of water freezing faster.
the phenomenon of snowSnow is water that falls from clouds that have been frozen into a solid and like rain or a crystallized form of water ice that formed from a variety of snowflakes. Shape snowflakes that there are different kinds, depending on the temperature of the surrounding air when formed. In the world, the land of snow is common in subtropical and temperate climates.Snow is formed from water vapor that is assembled in the atmosphere, water vapor cools collection to the point of condensation and agglomerate to form clouds. The clumps of water vapor floating in the air because of its mass is much lighter than air underneath. After a column of steam continued to grow and the heavier mass, underneath the air can no longer hold it and wadded it crashed. If the air temperature is quite cold underneath, before falling in the form of lumps of ice crystals (snow).Usually the air temperature just below the cloud is below zero degrees Celsius. However, low temperature alone is not enough to create snow. When particles of pure water is in contact with air, pure water is contaminated by other particles. There are certain particles that function accelerates the freezing phase, so that the pure water quickly become ice crystals.Pollutant particles involved in this process is called nukleator. In addition serves to accelerate the freezing phase, nukleator also serves as an adhesive between water vapor. Particles of water (which is not pure anymore) joined with other water particles to form larger crystals. If the air temperature is not to melt the ice crystals, ice crystals will fall to the ground into the snow. If the air temperature to melt the crystalline water, the ice crystals to the ground in the form of regular rain water.
Snow has these characteristics:
Snow crystals are formed from steam directly into a solid (frozen) in the cloud,
The shape of snow crystals usually hexagon.
The shape of snow crystals depends on the temperature and humidity.
Flurries of snow crystals formed from 2-200,
Actually snow colorless. Because it consists of snow crystals that form a prism, snow refract light into all the colors that the eye sees as white. Color snow could change if contaminated with dust that fly into the atmosphere. For example the local soil is red, snow reddish or pink.In some cases, the snow can be used for activities such as skiing, playing snowshoes, up to the sled with the dogs as pullers. In a very large number, the snow can damage the basic infrastructure such as electricity, telephone and gas lines that are an essential part of human life. In addition to being an important part for humans, Snow also has a negative impact on the health of one's body, such as blood pressure increases and the skin becomes dry.The occurrence of snow is one of the most amazing natural phenomenon and should be studied in order to uncover the secrets of nature contained therein. Environment which was considered impossible for the survival of living things turned out to be a habitat for some creatures living in it. Snow, who has a temperature below zero degrees Celsius become the foundation for some living creatures, one of which is algae.The form of snow is very varied, and are not equal to each other even though matched. It is really merpakan an amazing natural phenomenon.The process of snow depends on the environment that triggers the formation of the snow, but the principle is the same, namely: the fall of the water from the cloud that has been frozen into a solid like rain.
X . III
How to get energy from falling snow?
that the only way people harvest energy from snow is through hydroelectric dams.
Are there any other viable options?
Some ideas I've thought of:
-Spinners facing the sky that spin when weight falls on them
-artificial tree branches that hold a certain amount of snow and then get energy from the snap back.
The short answer is no, not really. There is not much useful energy
that can be extracted from snow.
Consider the principle of conservation of energy, which states that
energy cannot be created, it is just converted from one state to
another. What this means is that energy is not really 'created', it is
actually just captured from a high-energy source. The amount of energy
you can use/capture depends on converting something from a
higher-energy state to a lower-energy state.
A simple example is a spring. When the spring is compressed, it is at a
high-energy state. When you release it, it can transfer that energy to
something else (for example, to launch a ball into the air). But
afterward, the expanded spring is in a low-energy state. This is an
example of mechanical energy. You can also capture energy from
high-temperature materials - like steam. Steam is an example of
thermal energy.
Getting more into chemistry, in order to extract usable energy from a
material, you have to convert a high-energy material to a lower-energy
state. For example, in a combustion engine, gasoline is converted to
carbon dioxide and water. This is an example of chemical energy. There
is a lot of energy stored in the chemical bonds in gasoline, and much
less energy stored in the chemical bonds of carbon dioxide and water.
The energy that we can capture is in the difference between the high
energy state and the low energy state.
In the case of snow, there is not much of a lower-energy state that
snow can move toward. There is not a lot of energy stored in the bonds
of water, and it is at a low temperature, and there is not much
mechanical work to capture from it.
Perhaps the best example I can think of to use snow for energy might
be a little more indirect than you were thinking. The idea is to use
snow that is on a mountain. Because the snow on a mountain is at high
elevation, it has "potential energy" due to its height. When the snow
melts in spring, the water flows down the mountain, and that water
flow can be used to generate electricity (hydroelectric power).
However, I do not know of a way of using snow directly (as a material)
to generate power.
Snow as a Water Source
Knowledge Nuggets
- After a short adaptation period, pregnant beef cows will consume snow in amounts equivalent to the water intake of cows receiving liquid water. Through extensive testing in the early 1980's, the University of Alberta found insignificant differences in cow performance or body stress levels when asked to eat snow as their sole water source.
- Wintering beef cows are able to consume snow as their sole water source given that the snow is in a form that the cows can easily eat. The snow must be soft and friable so that the cows can lick a significant quantity into their mouths for melting.
- Once cows are used to consuming snow as their sole water source, they will consume small amounts of snow throughout the day during their free time. Cows can be seen licking snow before, during and after their primary feeding times.
- Although it takes ten times the amount of energy to melt a gram of snow from it's solid state to it's liquid state compared to heating the similar quantity of a liquid one degree Celsius, the key to energy use difference is found in the rate of consumption. Cows eating snow take all day to do so. Cows drinking cold trough water will consume their daily needs within minutes.
- Because of the slow and ongoing process of eating and melting snow, cows in effect use their waste heat to melt their snow. Because the melting process is such a slow process, the cow's body temperature never drops below it's critical point. Hence, the body's metabolism never needs to kick in to raise it's temperature as it does when a cow drinks large quantities of near freezing water from a waterer.
- The best indicator of whether a herd is getting enough water from melting snow is to monitor feed consumption. As long as feed consumption is adequate and consistent from day to day, the cows are getting enough water from the snow. Should feed consumption drastically drop over a short time period, water shortage may be the cause.
- The biggest stress for cattle eating snow is the transition period. Cattle that have never needed to eat snow and have only consumed water will vocalize to show their discontent. Following a day or two of discontent, the herd soon learns from the early learners that snow can be licked with positive results.
- Once cattle have learned to eat snow, the transition period is much shorter. Eventually cattle will eat snow without little discontent.
- Once cattle are accustomed to eating snow, they will often stay out in the fields where the feed is placed rather than walk home for water. The observation is that animals find it easier to eat snow rather that expend the energy to walk home to drink water.

snow flake
Snow pertains to frozen crystalline water throughout its life cycle, starting when it precipitates from clouds and accumulates on surfaces, then metamorphoses in place, and ultimately melts, slides or sublimates away. Snowstorms organize and develop by feeding on sources of atmospheric moisture and cold air. Snowflakes nucleate around particles in the atmosphere by attracting supercooled water droplets, which freeze in hexagonal-shaped crystals. Snowflakes take on a variety of shapes, basic among these are platelets, needles, columns and rime. As snow accumulates into a snowpack, it may blow into drifts. Over time, accumulated snow metamorphoses, by sintering, sublimation and freeze-thaw. Where the climate is cold enough for year-to-year accumulation, a glacier may form. Otherwise, snow typically melts, seasonally, and causes runoff into streams and rivers and recharging groundwater.
Major snow-prone areas include the polar regions, the upper half of the Northern Hemisphere and mountainous regions worldwide with sufficient moisture and cold temperatures. In the Southern Hemisphere, snow is confined primarily to mountainous areas, apart from Antarctica.
Snow affects such human activities as transportation: creating the need for keeping roadways, wings, and windows clear; agriculture: providing water to crops and safeguarding livestock, and such sports as skiing, snowboarding, and snowmachine travel. Snow affects ecosystems, as well, by providing an insulating layer during winter under which plants and animals are able to survive the cold.

Animation of snowfall areas (blue) in a mid-latitude cyclone. Icing is pink and rain is green.
Precipitation
Snow develops in clouds that themselves are part of a larger weather system. The physics of snow crystal development in clouds results from a complex set of variables that include moisture content and temperatures. The resulting shapes of the falling and fallen crystals can be classified into a number of basic shapes and combinations, thereof. Occasionally, some plate-like, dendritic and stellar-shaped snowflakes can form under clear sky with a very cold temperature inversion present.Cloud formation
Snow clouds usually occur in the context of larger weather systems, the most important of which is the low pressure area, which typically incorporate warm and cold fronts as part of their circulation. Two additional and locally productive sources of snow are lake-effect (also sea-effect) storms and elevation effects, especially in mountains.Low pressure areas
Animation of snowfall areas (blue) in a mid-latitude cyclone. Icing is pink and rain is green.
Fronts
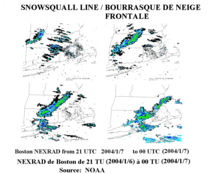
Frontal snowsquall moving toward Boston, Massachusetts, United States
A warm front can produce snow for a period, as warm, moist air overrides below-freezing air and creates precipitation at the boundary. Often, snow transitions to rain in the warm sector behind the front.
Lake and ocean effects
Cold northwesterly wind over Lake Superior and Lake Michigan creating lake-effect snowfall.
The same effect also occurs over bodies of salt water, when it is termed ocean-effect or bay-effect snow. The effect is enhanced when the moving air mass is uplifted by the orographic influence of higher elevations on the downwind shores. This uplifting can produce narrow but very intense bands of precipitation, which deposit at a rate of many inches of snow each hour, often resulting in a large amount of total snowfall.
The areas affected by lake-effect snow are called snowbelts. These include areas east of the Great Lakes, the west coasts of northern Japan, the Kamchatka Peninsula in Russia, and areas near the Great Salt Lake, Black Sea, Caspian Sea, Baltic Sea, and parts of the northern Atlantic Ocean.
Mountain effects
Orographic or relief snowfall is caused when masses of air pushed by wind are forced up the side of elevated land formations, such as large mountains. The lifting of air up the side of a mountain or range results in adiabatic cooling, and ultimately condensation and precipitation. Moisture is removed by orographic lift, leaving drier, warmer air on the descending, leeward side. The resulting enhanced productivity of snow fall and the decrease in temperature with elevation means that snow depth and seasonal persistence of snowpack increases with elevation in snow-prone areas.Cloud physics
Freshly fallen snowflakes.
Snow crystals form when tiny supercooled cloud droplets (about 10 μm in diameter) freeze. These droplets are able to remain liquid at temperatures lower than −18 °C (0 °F), because to freeze, a few molecules in the droplet need to get together by chance to form an arrangement similar to that in an ice lattice. Then the droplet freezes around this "nucleus". In warmer clouds an aerosol particle or "ice nucleus" must be present in (or in contact with) the droplet to act as a nucleus. Ice nuclei are very rare compared to that cloud condensation nuclei on which liquid droplets form. Clays, desert dust and biological particles can be nuclei. Artificial nuclei include particles of silver iodide and dry ice, and these are used to stimulate precipitation in cloud seeding.
Once a droplet has frozen, it grows in the supersaturated environment—one where air is saturated with respect to ice when the temperature is below the freezing point. The droplet then grows by diffusion of water molecules in the air (vapor) onto the ice crystal surface where they are collected. Because water droplets are so much more numerous than the ice crystals due to their sheer abundance, the crystals are able to grow to hundreds of micrometers or millimeters in size at the expense of the water droplets by the Wegener–Bergeron–Findeisen process. The corresponding depletion of water vapor causes the ice crystals to grow at the droplets' expense. These large crystals are an efficient source of precipitation, since they fall through the atmosphere due to their mass, and may collide and stick together in clusters, or aggregates. These aggregates are snowflakes, and are usually the type of ice particle that falls to the ground. Although the ice is clear, scattering of light by the crystal facets and hollows/imperfections mean that the crystals often appear white in color due to diffuse reflection of the whole spectrum of light by the small ice particles.
Classification of snowflakes
An early classification of snowflakes by Israel Perkins Warren.
Nakaya developed a crystal morphology diagram, relating crystal shapes to the temperature and moisture conditions under which they formed, which is summarized in the following table.
| Temperature range | Saturation range | Types of snow crystal | |||
|---|---|---|---|---|---|
| °C | °F | g/m3 | oz/cu yd | below saturation | above saturation |
| 0 to −3.5 | 32 to 26 | 0.0 to 0.5 | 0.000 to 0.013 | Solid plates | Thin plates Dendrites |
| −3.5 to −10 | 26 to 14 | 0.5 to 1.2 | 0.013 to 0.032 | Solid prisms Hollow prisms | Hollow prisms Needles |
| −10 to −22 | 14 to −8 | 1.2 to 1.4 | 0.032 to 0.038 | Thin plates Solid plates | Sectored plates Dendrites |
| −22 to −40 | −8 to −40 | 1.2 to 0.1 | 0.0324 to 0.0027 | Thin plates Solid plates | Columns Prisms |
Magono and Lee devised a classification of freshly formed snow crystals that includes 80 distinct shapes. They documented each with micrographs.
Accumulation
An animation of seasonal snow changes, based on satellite imagery.
Snow events
Snow flurry, snow storm and blizzard describe snow events of progressively greater duration and intensity. A blizzard is a weather condition involving snow and has varying definitions in different parts of the world. In the United States, a blizzard occurs when two conditions are met for a period of three hours or more: A sustained wind or frequent gusts to 35 miles per hour (56 km/h), and sufficient snow in the air to reduce visibility to less than 0.4 kilometers (0.25 mi). In Canada and the United Kingdom, the criteria are similar.[35][36] While heavy snowfall often occurs during blizzard conditions, falling snow is not a requirement, as blowing snow can create a ground blizzard.Snowstorm intensity may be categorized by visibility and depth of accumulation. Snowfall's intensity is determined by visibility, as follows:
- Light: visibility greater than 1 kilometer (0.6 mi)
- Moderate: visibility restrictions between 0.5 and 1 kilometer (0.3 and 0.6 mi)
- Heavy: visibility is less than 0.5 kilometers (0.3 mi)
Distribution
Glaciers with their permanent snowpacks cover about 10% of the earth's surface, while seasonal snow covers about nine percent, mostly in the Northern Hemisphere, where seasonal snow covers about 40 million square kilometres (15×106 sq mi), according to a 1987 estimate. A 2007 estimate of snow cover over the Northern Hemisphere suggested that, on average, snow cover ranges from a minimum extent of 2 million square kilometres (0.77×106 sq mi) each August to a maximum extent of 45 million square kilometres (17×106 sq mi) each January or nearly half of the land surface in that hemisphere. A study of Northern Hemisphere snow cover extent for the period 1972–2006 suggests a reduction of 0.5 million square kilometres (0.19×106 sq mi) over the 35-year period.Records
The following are world records, regarding snowfall and snowflakes:- Highest seasonal total snowfall – The world record for the highest seasonal total snowfall was measured in the United States at Mount Baker Ski Area, outside of the town Bellingham, Washington during the 1998–1999 season. Mount Baker received 2,896 cm (95.01 ft) of snow, thus surpassing the previous record holder, Mount Rainier, Washington, which during the 1971–1972 season received 2,850 cm (93.5 ft) of snow.
- Highest seasonal average annual snowfall – The world record for the highest average annual snowfall is 1,764 cm (57.87 ft), measured in Sukayu Onsen, Japan for the period of 1981–2010.
- Largest snowflakes – Guinness World Records list the world's largest snowflakes as those of January 1887 at Fort Keogh, Montana; allegedly one measured 38 cm (15 in) wide.
Metamorphosis
Fresh snow beginning to metamorphose: The surface shows wind packing and sastrugi. In the foreground are hoar frost crystals, formed by refrozen water vapor emerging to the cold surface.
Seasonal snowpack
Over the course of time, a snowpack may settle under its own weight until its density is approximately 30% of water. Increases in density above this initial compression occur primarily by melting and refreezing, caused by temperatures above freezing or by direct solar radiation. In colder climates, snow lies on the ground all winter. By late spring, snow densities typically reach a maximum of 50% of water. Snow that persists into summer evolves into névé, granular snow, which has been partially melted, refrozen and compacted. Névé has a minimum density of 500 kilograms per cubic metre (31 lb/cu ft), which is roughly half of the density of liquid water.Firn
Firn—metamorphosed multi-year snow.
Movement
There are four main mechanisms for movement of deposited snow: drifting of unsintered snow, avalanches of accumulated snow on steep slopes, snowmelt during thaw conditions, and the movement of glaciers after snow has persisted for multiple years and metamorphosed into glacier ice.Drifting
Snow drifts forming around downwind obstructions.
Avalanche
Main article: Avalanche
A powder snow avalanche.
- Slab avalanches occur in snow that has been deposited, or redeposited by wind. They have the characteristic appearance of a block (slab) of snow cut out from its surroundings by fractures. These account for most back-country fatalities.
- Powder snow avalanches result from a deposition of fresh dry powder and generate a powder cloud, which overlies a dense avalanche. They can exceed speeds of 300 kilometres per hour (190 mph), and masses of 10,000,000 tonnes (9,800,000 long tons; 11,000,000 short tons); their flows can travel long distances along flat valley bottoms and even uphill for short distances.
- Wet snow avalanches are a low-velocity suspension of snow and water, with the flow confined to the surface of the pathway. The low speed of travel is due to the friction between the sliding surface of the pathway and the water saturated flow. Despite the low speed of travel (~10 to 40 kilometres per hour (6 to 25 mph)), wet snow avalanches are capable of generating powerful destructive forces, due to the large mass, and density.
Snowmelt
Snowmelt-induced flooding of the Red River of the North in 1997.
Glaciers
Glaciers form where the accumulation of snow and ice exceeds ablation. The area in which an alpine glacier forms is called a cirque (corrie or cwm), a typically armchair-shaped geological feature, which collects snow and where the snowpack compacts under the weight of successive layers of accumulating snow, forming névé. Further crushing of the individual snow crystals and reduction of entrapped air in the snow turns it into 'glacial ice'. This glacial ice will fill the cirque until it 'overflows' through a geological weakness or vacancy, such as the gap between two mountains. When the mass of snow and ice is sufficiently thick, it begins to move due to a combination of surface slope, gravity and pressure. On steeper slopes, this can occur with as little as 15 m (50 ft) of snow-ice.Snow science
Scientists study snow at a wide variety of scales that include the physics of chemical bonds and clouds; the distribution, accumulation, metamorphosis, and ablation of snowpacks; and the contribution of snowmelt to river hydraulics and ground hydrology. In doing so, they employ a variety of instruments to observe and measure the phenomena studied. Their findings contribute to knowledge applied by engineers, who adapt vehicles and structures to snow, by agronomists, who address the availability of snowmelt to agriculture, and those, who design equipment for sporting activities on snow. Scientists develop and others employ snow classification systems that describe its physical properties at scales ranging from the individual crystal to the aggregated snowpack. A sub-specialty is avalanches, which are of concern to engineers and outdoors sports people, alike.Snow science addresses how snow forms, its distribution, and processes affecting how snowpacks change over time. Scientists improve storm forecasting, study global snow cover and its effect on climate, glaciers, and water supplies around the world. The study includes physical properties of the material as it changes, bulk properties of in-place snow packs, and the aggregate properties of regions with snow cover. In doing so, they employ on-the-ground physical measurement techniques to establish ground truth and remote sensing techniques to develop understanding of snow-related processes over large areas.
Measurement and classification
Snow pit on the surface of a glacier, profiling snow properties where
the snow becomes increasingly dense with depth as it metamorphoses
towards ice.
| Subclass | Shape | Physical process |
|---|---|---|
| Graupel | Heavily rimed particles, spherical, conical, hexagonal or irregular in shape | Heavy riming of particles by accretion of supercooled water droplets |
| Hail | Laminar internal structure, translucent or milky glazed surface | Growth by accretion of supercooled water, size: >5 mm |
| Ice pellets | Transparent, mostly small spheroids | Freezing of raindrops or refreezing of largely melted snow crystals or snowflakes (sleet). Graupel or snow pellets encased in thin ice layer (small hail). Size: both 5 mm |
| Rime | Irregular deposits or longer cones and needles pointing into the wind | Accretion of small, supercooled fog droplets frozen in place. Thin breakable crust forms on snow surface if process continues long enough. |
It also has a more extensive classification of deposited snow than those that pertain to airborne snow. The categories include both natural and man-made snow types, descriptions of snow crystals as they metamorphose and melt, the development of hoar frost in the snow pack and the formation of ice therein. Each such layer of a snowpack differs from the adjacent layers by one or more characteristics that describe its microstructure or density, which together define the snow type, and other physical properties. Thus, at any one time, the type and state of the snow forming a layer have to be defined because its physical and mechanical properties depend on them. Physical properties include microstructure, grain size and shape, snow density, liquid water content, and temperature.
Satellite data
Remote sensing of snowpacks with satellites and other platforms typically includes multi-spectral collection of imagery. Multi-faceted interpretation of the data obtained allows inferences about what is observed. The science behind these remote observations has been verified with ground-truth studies of the actual conditions.Satellite observations record a decrease in snow-covered areas since the 1960s, when satellites when satellite observations began. In some areas, including China, snow cover has increased. In some regions such as China, a trend of increasing snow cover has been observed from 1978 to 2006. These changes are attributed to global climate change, which may lead to earlier melting and less aea coverage. However, in some areas there may be an increase in snow depth because of higher temperatures for latitudes north of 40°. For the Northern Hemisphere as a whole the mean monthly snow-cover extent has been decreasing by 1.3% per decade.
The most frequently used methods to map and measure snow extent, snow depth and snow water equivalent employ multiple inputs on the visible–infrared spectrum to deduce the presence and properties of snow. The National Snow and Ice Data Center (NSIDC) uses the reflectance of visible and infrared radiation to calculate a normalized difference snow index, which is a ratio of radiation parameters that can distinguish between clouds and snow. Other researchers have developed decision trees, employing the available data to make more accurate assessments. One challenge to this assessment is where snow cover is patchy, for example during periods of accumulation or ablation and also in forested areas. Cloud cover inhibits optical sensing of surface reflectance, which has led to other methods for estimating ground conditions underneath clouds. For hydrological models, it is important to have continuous information about the snow cover. Passive microwave sensors are especially valuable for temporal and spatial continuity because they can map the surface beneath clouds and in darkness. When combined with reflective measurements, passive microwave sensing greatly extends the inferences possible about the snowpack.
Models
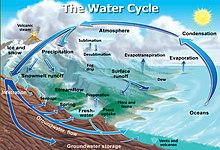
Snowfall and snowmelt are parts of the Earth's water cycle.
Global climate change models (GCMs) incorporate snow as a factor in their calculations. Some important aspects of snow cover include its albedo (reflectivity of incident radiation, including light) and insulating qualities, which slow the rate of seasonal melting of sea ice. As of 2011, the melt phase of GCM snow models were thought to perform poorly in regions with complex factors that regulate snow melt, such as vegetation cover and terrain. These models typically derive snow water equivalent (SWE) in some manner from satellite observations of snow cover. The International Classification for Seasonal Snow on the Ground defines SWE as "the depth of water that would result if the mass of snow melted completely".
Given the importance of snowmelt to agriculture, hydrological runoff models that include snow in their predictions address the phases of accumulating snowpack, melting processes, and distribution of the meltwater through stream networks and into the groundwater. Key to describing the melting processes are solar heat flux, ambient temperature, wind, and precipitation. Initial snowmelt models used a degree-day approach that emphasized the temperature difference between the air and the snowpack to compute snow water equivalent, SWE. More recent models use an energy balance approach that take into account the following factors to compute Qm, the energy available for melt. This requires measurement of an array of snowpack and environmental factors to compute six heat flow mechanisms that contribute to Qm.
Effects on human activity
Snow effects human activity in four major areas, transportation, agriculture, structures, and sports. Most transportation modes are impeded by snow on the travel surface. Agriculture often relies on snow as a source of seasonal moisture. Structures may fail under snow loads. Humans find a wide variety of recreational activities in snowy landscapes.Transportation
See also: Snowplow
Snow affects the rights of way of highways, airfields and railroads. They share a common tool for clearing snow, the snow plow.
However, the application is different in each case—whereas roadways
employ anti-icing chemicals to prevent bonding of ice, airfields may
not; railroads rely on abrasives to enhance traction on tracks.Highway
Traffic stranded in a 2011 Chicago snowstorm.
The dominant effect of snow on vehicle contact with the road is diminished friction. This can be improved with the use of snow tires, which have a tread designed to compact snow in a manner that enhances traction. However, the key to maintaining a roadway that can accommodate traffic during and after a snow event is an effective anti-icing program that employs both chemicals and plowing. The FHWA Manual of Practice for an Effective Anti-icing Program emphasizes "anti-icing" procedures that prevent the bonding of snow and ice to the road. Key aspects of the practice include: understanding anti-icing in light of the level of service to be achieved on a given roadway, the climatic conditions to be encountered, and the different roles of deicing, anti-icing, and abrasive materials and applications, and employing anti-icing "toolboxes", one for operations, one for decision-making and another for personnel. The elements to the toolboxes are:
- Operations – Addresses the application of solid and liquid chemicals, using various techniques, including prewetting of chloride-salts. It also addresses plowing capability, including types of snowplows and blades used.
- Decision-making – Combines weather forecast information with road information to assess the upcoming needs for application of assets and the evaluation of treatment effectiveness with operations underway.
- Personnel – Addresses training and deployment of staff to effectively execute the anti-icing program, using the appropriate materials, equipment and procedures.
Snow fences, constructed upwind of roadways control snow drifting by causing windblown, drifting snow to accumulate in a desired place. They are also used on railways. Additionally, farmers and ranchers use snow fences to create drifts in basins for a ready supply of water in the spring.[61][62]
Aviation
Deicing an aircraft during a snow event.
Aircraft are able to fly through snowstorms under Instrument Flight Rules. Prior to takeoff, during snowstorms they require deicing fluid to prevent accumulation and freezing of snow and other precipitation on wings and fuselages, which may compromise the safety of the aircraft and its occupants. In flight, aircraft rely on a variety of mechanisms to avoid rime and other types of icing in clouds, these include pulsing pneumatic boots, electro-thermal areas that generate heat, and fluid deicers that bleed onto the surface.
Rail
Railroads have traditionally employed two types of snow plows for clearing track, the wedge plow, which casts snow to both sides, and the rotary snowplow, which is suited for addressing heavy snowfall and casting snow far to one side or the other. Prior to the invention of the rotary snowplow ca. 1865, it required multiple locomotives to drive a wedge plow through deep snow. Subsequent to clearing the track with such plows, a "flanger" is used to clear snow from between the rails that are below the reach of the other types of plow. Where icing may affect the steel-to-steel contact of locomotive wheels on track, abrasives (typically sand) have been used to provide traction on steeper uphills.Railroads employ snow sheds—structures that cover the track—to prevent the accumulation of heavy snow or avalanches to cover tracks in snowy mountainous areas, such as the Alps and the Rocky Mountains.
- Snowplows for different transportation modes
-
Trucks plowing snow on a highway in Indiana.
Agriculture
Satellite view of the Indus River, showing snow in the Himalayas, which
feeds it, and green areas that draw on it for irrigation.
Structures
Snow accumulation on building roofs
Roofs
Icings resulting from water leaking through the roof due to an ice dam, caused by snowmelt on the roof.
Snow loads – The Minimum Design Loads for Buildings and Other Structures gives guidance on how to translate the following factors into roof snow loads:
- Ground snow loads
- Exposure of the roof
- Thermal properties of the roof
- Shape of the roof
- Drifting
- Importance of the building
Icings – Roofs must also be designed to avoid ice dams, which result from meltwater running under the snow on the roof and freezing at the eave. Ice dams on roofs form when accumulated snow on a sloping roof melts and flows down the roof, under the insulating blanket of snow, until it reaches below freezing temperature air, typically at the eaves. When the meltwater reaches the freezing air, ice accumulates, forming a dam, and snow that melts later cannot drain properly through the dam. Ice dams may result in damaged building materials or in damage or injury when the ice dam falls off or from attempts to remove ice dams. The melting results from heat passing through the roof under the highly insulating layer of snow.
Effects on ecosystems
Algae, Chlamydomonas nivalis, that thrive in snow form red areas in the suncups on this snow surface.
Plant life
Snow interacts with vegetation in two principal ways, vegetation can influence the deposition and retention of snow and, conversely, the presence of snow can affect the distribution and growth of vegetation. Tree branches, especially of conifers intercept falling snow and prevent accumulation on the ground. Snow suspended in trees ablates more rapidly than that on the ground, owing to its greater exposure to sun and air movement. Trees and other plants can also promote snow retention on the ground, which would otherwise be blown elsewhere or melted by the sun. Snow affects vegetation in several ways, the presence of stored water can promote growth, yet the annual onset of growth is dependent on the departure of the snowpack for those plants that are buried beneath it. Furthermore, avalanches and erosion from snowmelt can scour terrain of vegetation.Animal life
Arctic fox, a predator of smaller animals that live beneath the snow
Small vertebrates are active beneath the snow. Among vertebrates, alpine salamanders are active in snow at temperatures as low as −8 °C (18 °F); they burrow to the surface in springtime and lay their eggs in melt ponds. Among mammals, those that remain active are typically smaller than 250 grams (8.8 oz). Omnivores are more likely to enter a torpor or be hibernators, whereas herbivores are more likely to maintain food caches beneath the snow. Voles store up to 3 kilograms (6.6 lb) of food and pikas up to 20 kilograms (44 lb). Voles also huddle in communal nests to benefit from one another's warmth. On the surface, wolves, coyotes, foxes, lynx, and weasels rely on these subsurface dwellers for food and often dive into the snowpack to find them.
Extraterrestrial snow
Extraterrestrial "snow" includes water-based precipitation, but also precipitation of other compounds prevalent on other planets and moons in the Solar System. Examples are:- On Mars, observations of the Phoenix Mars lander reveal that water-based snow crystals occur at high latitudes. Additionally, carbon dioxide precipitates from clouds during the Martian winters at the poles and contributes to a seasonal deposit of that compound, which is the principal component of that planet's ice caps.
- On Venus, observations from the Magellan spacecraft reveal the presence a metallic substance, which precipitates as "Venus snow" and leaves a highly reflective substance at the tops of Venus's highest mountain peaks resembling terrestrial snow. Given the high temperatures on Venus, the leading candidates for the precipitate are lead sulfide and bismuth sulfide.
- On Saturn's moon, Titan, Cassini–Huygens spacecraft observations suggest the presence of methane or some other form of hydrocarbon-based crystalline deposits.