
In a laboratory controlled scientific investigation, a key biological marker has been identified, quantified, and directly correlated with the application of acupuncture. Acupuncture successfully down regulates a pro inflammatory biochemical (tumor necrosis factor alpha), which results in anti-inflammatory responses. In addition, the researchers have mapped the neural pathways by which acupuncture signaling stimulates anti-inflammatory effects.
ELECTRO-ACUPUNCTURE
GENERAL PRINCIPLES
Electro-acupuncture, the application of a pulsating electrical current to acupuncture needles as a means of stimulating the acupoints, The procedure for electro-acupuncture is to insert the acupuncture needle as would normally be done, attain the qi reaction by hand manipulation, and then attach an electrode to the needle to provide continued stimulation. The benefits of using electrical stimulation are:
- It substitutes for prolonged hand maneuvering. This helps assure that the patient gets the amount of stimulation needed, because the practitioner may otherwise pause due to fatigue. Electro-acupuncture may also help reduce total treatment time by providing the continued stimulus. During electro-acupuncture, the practitioner can attend to other patients.
- It can produce a stronger stimulation, if desired, without causing tissue damage associated with twirling and lifting and thrusting the needle. Strong stimulation may be needed for difficult cases of neuralgia or paralysis.
- It is easier to control the frequency of the stimulus and the amount of stimulus than with hand manipulation of the needles.
The main disadvantage of electrical stimulation of acupuncture needles is the lack of direct practitioner participation in this aspect of acupuncture therapy and the associated limited opportunity for the practitioner to respond to changes that are taking place during treatment. However, for practitioners who, after inserting and initially stimulating the needles, normally leave the patient to rest undisturbed without performing prolonged needle manipulation, electro-acupuncture can provide a significant benefit: replacing the missing stimulus .
Although electro-acupuncture may be used as a component of nearly all acupuncture treatments that require manipulation of the needles, according to the Chinese literature, especially good results are expected from electro-acupuncture treatment of neurological diseases, including chronic pain, spasm, and paralysis. In patients with serious cardiac diseases, however, the method should be used with caution. It is generally recommended to avoid placing electrodes near the heart, as the heart can respond adversely to electrical impulses, and the path between any two electrodes should not cross the heart area, despite the low current that is used. Some have suggested avoiding placing electrodes to needles on both sides of the spinal cord . because of the possible effect of the electrical stimulus on the nervous system. Points are generally selected in pairs for electrical pulse stimulation, with 1-3 pairs at one time, and the pairs are usually on the same side of the body.
THE USE OF ELECTRICAL STIMULATION DEVICES
The electro-acupuncture device is not intended to provide a significant current between the acupuncture needles. Rather, it delivers about 10-80 milliamps depending upon the selected setting. But, it will provide a significant voltage: 40-80 volts, which is the basis for the patient response. In the commonly-used portable battery devices, this is accomplished by boosting the voltage output of the battery, such as raising the voltage from 9 volts to 45 volts. Many of the devices have an AC adapter to avoid frequent replacement of batteries, and this involves a substantial step-down of both voltage and amperage. There is virtually no current transmitted through the body, but there is enough voltage stimulus for the patient to feel it; often this will be a pulsating sensation because of the intention of using a waveform that is perceptible.
Duration of standard treatment with electro-acupuncture is usually 10-20 minutes and rarely exceeds 30 minutes. The electrical pulsing stimulus is used in a few cases for an hour or more, especially for difficult to treat neurological disorders. During the stimulation period, the patient may become adapted to the stimulus (this will typically happen after the first minute or two), with a gradual decline in response. The electrical output should then be adjusted in frequency and/or intensity to resume the sensation. Variable frequency output of the electro-acupuncture device is sometimes utilized in an attempt to circumvent this adaptation.
Electro-acupuncture is normally administered with alternating current. Therefore, the two electrodes in any pair are equivalent, even if they are color coded to distinguish them. Some devices allow a direct current (non-alternating) setting, but the use of this has been discouraged, as mild adverse effects might occur if the pulsing of the current ceases for any reason (i.e., device defect). Further, it has been suggested, though it remains to be proven, that the adaptation to the direct current may be more rapid than to the alternating current. When it is said in electro-acupuncture literature that "the negative electrode is attached to what is considered the main point, while the positive electrode is attached to a secondary point," the statement has no relevance when using an alternating current.

Figure 1: An electro-acupuncture stimulator.
The device to be used for electro-acupuncture (see Figure 1 for modern example) must have good control over its voltage output to avoid excessive electrical stimulation, namely an unexpected higher voltage pulse that causes pain or muscular contraction, and to assure that the frequency and intensity is maintained as set by the practitioner. Informal testing of devices has showed that some are erratic in their output, so older devices, and those not produced with adequate quality control measures, are to be avoided. A device commonly used in China is the G6805 or G6805-2 electric stimulator.
The device should not be turned on until after the acupuncture needles are in place and the electrodes connected. All changes in the electrical stimulus should be carried out gradually. It is normal for the patient to experience responses such as rhythmic spasm or weak twitching of the muscle (frequently visible to the practitioner), as well as the usual "deqi" reactions of acupuncture therapy: sensation of numbness, distention, and/or heaviness. The stimulus intensity, set by a voltage-adjusting knob on the device, should be in the range between the minimum amount needed for the patient to sense its effect and the minimum amount that produces an uncomfortable reaction; care should be taken to limit the muscle twitching to a mild response. Areas that are particularly sensitive to electrical stimulation are the face and regions below the elbow and knee. These areas should be treated initially with a very low intensity voltage. Patients who have not had acupuncture previously should receive standard acupuncture first to assure that they tolerate the treatment well, before moving on to electro-acupuncture, which may yield a stronger sensation.
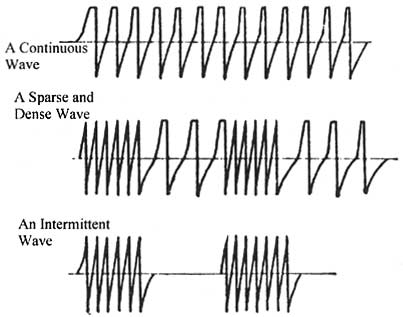
Figure 2: Samples of wave forms produced by electro-acupuncture devices.
The wave form (squared off or sharp; dense, sparse, mixed dense and sparse, or interrupted; see Figure 2 for sample outputs) can provide slightly different responses and must be tried for each patient to evaluate their suitability. Claims that one form is tonifying and another is dispersing may not be justified due to lack of adequate testing to support such differentiation of effects. A continuous wave (frequency doesn't change over time) is most similar to what acupuncturists in China attempt to administer by the manual method. The overall frequency of stimulus (either by continuous wave or pulses of dense waves) should be set similar to the frequency that would be used in manual stimulation by twirling or lifting/thrusting. Frequencies as high as 200 pulses per minute have been recommended for scalp acupuncture, with low frequencies being more commonly mentioned for body acupuncture (e.g., 50 or less). Different authors writing about electro-acupuncture present differing opinions on the ideal frequency for various desired effects. All the wave forms and frequencies are claimed to be of value in promoting circulation of qi and blood and alleviating various symptoms, particularly pain.
Although some theories have been developed regarding the mechanism of action of electro-acupuncture, there are no conclusive tests. The main function of electro-acupuncture, as evidenced by the discussions in several clinical reports in the Chinese medical literature, appears to be no more than pulsation by voltage spikes serving as stimulus replacing a rhythmic physical movement as stimulus at the site.
acupuncture literature shows that most clinical work with acupuncture is carried out with manual stimulus despite the ready availability of electro-acupuncture devices. In clinical trials where electro-acupuncture is used, there are few details, if any, reported about the technique, other than the duration of stimulus and the frequency of the electrical output. In one study of treatment of depression in which some explanation was given (5), the authors stated that "The intensity of stimulation was optimal when slight muscle twitching was visible, yet the patient was comfortable and the stimulus tolerable. The frequency of stimulus chosen was about 80-90 beats per minute. The duration of treatment was 1 hour each time, once a day (except Sundays) for 5 weeks, altogether 30 needlings." The authors expressed the belief, based on animal studies of electro-acupuncture, that the treatment would promote production of neurotransmitters that would alleviate the brain dysfunction. The same kind of biochemical response has been suggested as the mechanism for standard (non-electric stimulated) acupuncture.
SUMMARY
Electro-acupuncture is a convenient stimulation technique to be utilized with the same acupuncture points and the same number of treatments as with manual acupuncture. For busy practitioners and those who do not normally provide prolonged manual needle stimulation, electro-acupuncture may improve the clinical situation. In cases where intensive, high frequency, and prolonged treatments might be deemed essential, as with certain stubborn neurological disorders, electro-acupuncture may be the only means to provide effective daily treatments. Practitioners should consult literature accompanying the device they purchase regarding contraindications for electro-acupuncture and recommendations for using different pulse forms and frequencies, but should also be aware that there may be very limited basis for some of the statements that are made.
: Electric field around an acupuncture needle

Electro-Acupuncture
ELECTRO ACUPUNCTURE: HIGH POWER ELECTRO ACUPUNCTURE UNIT

Electronic Acupunctoscope
Multiple Electronic Acupunctoscope with Therapeutic Switch | ||
| ||
| WQ-6F Feature: | ||
| ||
|
| WQ-6F Operation: | |||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||
| Notes: The WQ-6F can emit 2 waveforms simultaneously, 1 from section A and 1 from section B. 7 channels do not interfere each other, but you can connect outputs in parallel or in succession to enhance stimulation for various purposes. When channels are connected in parallel, the output current is enhanced while the output voltage maintains the same. When channels are connected in succession, the output voltage is enhanced while the output current maintains the same. | |||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||
| Electronic Acupunctoscope Technical Description | |||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||
Features:
|
Operation
| |||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||
| Notes: The WQ-6F can emit 2 waveforms simultaneously, 1 from section A and 1 from section B. 7 channels do not interfere each other, but you can connect outputs in parallel or in succession to enhance stimulation for various purposes. When channels are connected in parallel, the output current is enhanced while the output voltage maintains the same. When channels are connected in succession, the output voltage is enhanced while the output current maintains the same. | ||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||


XO___XO Mechanisms of Acupuncture–Electroacupuncture on Persistent Pain
In the last decade, preclinical investigations of electroacupuncture mechanisms on persistent tissue injury (inflammatory), nerve injury (neuropathic), cancer, and visceral pain have increased. These studies show that electroacupuncture activates the nervous system differently in health than in pain conditions, alleviates both sensory and affective inflammatory pain, and inhibits inflammatory and neuropathic pain more effectively at 2 to 10 Hz than at 100 Hz. Electroacupuncture blocks pain by activating a variety of bioactive chemicals through peripheral, spinal, and supraspinal mechanisms. These include opioids, which desensitize peripheral nociceptors and reduce proinflammatory cytokines peripherally and in the spinal cord, and serotonin and norepinephrine, which decrease spinal N-methyl-d-aspartate receptor subunit GluN1 phosphorylation. Additional studies suggest that electroacupuncture, when combined with low dosages of conventional analgesics, provides effective pain management which can forestall the side effects of often-debilitating pharmaceuticals.
PAIN, a major health problem with serious social and economic consequences, costs the economy $560 to $635 billion annually in physician visits, analgesics, and loss of productivity.1 Conventional medical treatments are only moderately efficacious, and they often produce problematic side effects. Acupuncture/electroacupuncture, used in China and other Asian countries for the past 3,000 yr, represents a potentially valuable adjunct to existing pain-relief strategies.2 Approximately 2 million American adults used acupuncture in 20023 ; this increased to 3 million in 2007, with chronic pain being the most common reason for seeking acupuncture treatment. Concomitant with increasing use of the modality, research has been performed on acupuncture mechanisms, and data from these studies have accumulated.
On the basis of etiology, pain may be classified into tissue damage–induced inflammatory/nociception and nerve damage–induced neuropathy. The former is caused by a painful stimulus on nociceptors and the latter by a primary lesion or dysfunction in the nervous system. On the basis of origin, pain may also be classified as somatic or visceral. Notably, some pain, for example cancer-related pain, experienced by one third of patients receiving treatment for cancer and approximately two thirds of those with advanced cancers, is not easily classifiable. A variety of animal models have been used to study the effect and mechanisms of electroacupuncture on persistent pain (fig. 1). This review synthesizes these studies to give an overall picture of how electroacupuncture alleviates pain through peripheral and central mechanisms of the body and to show that a number of bioactive chemicals are involved in electroacupuncture inhibition of pain.
Inflammatory Pain Animal Models
The effect and mechanisms of acupuncture/electroacupuncture on persistent pain have been studied at peripheral, spinal, and supraspinal levels by using inflammatory pain animal models, most of which were produced by complete Freund’s adjuvant (CFA: inactivated and dried Mycobacterium and adjuvant) or carrageenan. Many bioactive chemicals are involved in electroacupuncture inhibition of pain (table 1).
Peripheral Mechanisms
Peripheral inflammatory cells–released opioids are involved in electroacupuncture inhibition of inflammatory pain. Studies in carrageenan-induced inflammatory pain rat models show that an intraplantar injection of naloxone or selective antagonists against μ- (d-Phe-Cys-Tyr-d-Trp-Orn-Thr-Pen-ThrNH2), δ- (naltrindole), or κ- (nor-Binaltorphimine) opioid receptors 1 h before electroacupuncture treatment at Zusanli (ST36; fig. 2) dosage-dependently blocked electroacupuncture-produced inhibition of mechanical hyperalgesia assessed through paw pressure threshold.4,5 Consistent with these results, intraplantar naloxone methiodide, a peripherally acting opioid receptor antagonist and an antibody against β-endorphin, eliminated electroacupuncture-produced inhibition of CFA-induced thermal hyperalgesia assessed with paw withdrawal latency (PWL) in response to radiant thermal stimuli.6 These data indicate that electroacupuncture induces release of endogenous opioids from lymphocytes, monocytes/macrophages, and granulocytes7,8 into inflamed skin. The opioids in turn activate receptors on peripheral nerve terminals to suppress nociception.
Electroacupuncture activates sympathetic nerve fibers to increase endogenous opioid in inflammatory site. Activation of sympathetic nerve fiber enhances the expression of intracellular adhesion molecule-1 in the blood vessels of inflamed tissue to promote migration of β-endorphin– and met-enkephalin–containing polymorphonuclear leukocytes and mononuclear cells in rats with CFA-induced hind paw inflammation.9 Furthermore, sympathetic neuron–derived norepinephrine stimulates adrenergic receptors on inflammatory cells to release β-endorphin, leading to inhibition of pain.10 Electroacupuncture activates sympathetic nerve fibers to inhibit pain11,12 although the exact mechanism is not clear. For instance, pretreatment with either 6-hydroxydopamine, a neurotoxin for sympathetic nerve endings, or the β-adrenoceptor antagonist propranolol significantly prevents electroacupuncture inhibition of carrageenan-induced thermal hyperalgesia.13 This electroacupuncture action on sympathetic nerves might enhance migration of opioid-containing cells to an inflammatory site, increasing the release of endogenous opioids.
Electroacupuncture also increases endogenous cannabinoid CB2 receptors (CB2R) to up-regulate opioids in inflamed skin tissue. At Huantiao (GB30) and Yanglingquan (GB34), the modality significantly increased proopiomelanocortin messenger RNA (mRNA) and β-endorphin levels in inflamed skin tissue as well as the percentage of β-endorphin-immunoreactive keratinocytes, macrophages, and T-lymphocytes.14 These effects were significantly attenuated by CB2R antagonist AM630 pretreatment. Interestingly, electroacupuncture also increased the levels of endogenous anandamide in inflamed tissue15 and the expression of CB2R on keratinocytes, macrophages, and T-lymphocytes in inflammation.16
In a recent study in the CFA-induced inflammatory pain rat model, electroacupuncture significantly increased PWL and mechanical threshold assessed with von Frey filaments and significantly decreased tumor necrosis factor-α (TNF-α), interleukin (IL)-1β, and IL-6 expression in inflamed skin.17 Moreover, electroacupuncture’s inhibition of pain was significantly attenuated by the CB2R antagonist AM630, as was cytokine expression.17 Because proinflammatory cytokines facilitate nociception18 and morphine inhibits cytokine release from peripheral blood mononuclear cell cultures,19,20 electroacupuncture mitigates pain by up-regulating endogenous anandamide, which activates CB2R to promote opioid production, consequently blocking cytokine release to inhibit pain.
Electroacupuncture inhibition of cyclooxygenase-2 (COX-2) might increase levels of endogenous anandamide. The modality activated the hypothalamus–pituitary–adrenal axis to significantly increase plasma corticosterone levels in a CFA-inflammatory pain rat model21,22 and significantly down-regulated carrageenan-induced expression of COX-1, COX-2 mRNA, and their proteins.23 It is known that the endocannabinoids anandamide and 2-arachidonoyl glycerol are metabolized by fatty acid amide hydrolase, monoacylglycerol lipase, and COX-2.24 Thus electroacupuncture-induced corticosterone might inhibit COX-2 to interfere with endocannabinoid metabolism, resulting in their escalation at inflammatory sites and leading to an increase in opioids.
Corticotrophin-releasing factor (CRF) and prostaglandin E2 (PGE2) are also involved in electroacupuncture analgesia. For instance, an intraplantar CRF antagonist also prevented electroacupuncture inhibition of inflammatory pain.6,25 CRF is known to block pain by stimulating the release of opioids from immune cells.26,27 Thus electroacupuncture might induce skin fibroblasts to release CRF, which in turn stimulates opioid release to inhibit pain. Electroacupuncture decreased carrageenan-induced PGE2 in inflammatory paws23 ; because PGE2 receptor activation of peripheral nociceptors contributes to pain, this ability to inhibit PGE2 might also be a factor in electroacupuncture’s effect on pain.28
Manual acupuncture or a local injection of the adenosine A1 receptor agonist 2-chloro-N(6)-cyclopentyladenosine at acupoint ST36 significantly inhibits mechanical allodynia and thermal hyperalgesia in wild-type but not in adenosine A1 receptor knockout mice with inflammatory and neuropathic pain.29 In addition, both interventions suppress high-intensity stimulation-evoked field excitatory postsynaptic potentials in the anterior cingulate cortex (ACC). Acupuncture also significantly increased extracellular adenosine near ST36. The investigators concluded that acupuncture attenuated the pain by increasing local adenosine that acts on A1 receptors in sensory afferents of ascending nerve tracks.29
Collectively, these studies demonstrate that peripheral opioids play a central role in electroacupuncture inhibition of inflammatory pain by blocking proinflammatory cytokine release from polymorphonuclear leukocytes and mononuclear cells and by acting on peripheral opioid receptors to desensitize peripheral sensory nerves (fig. 3).
Fig. 3.
Mechanisms of electroacupuncture inhibition of inflammatory and neuropathic pain. Symbols + and − respectively represent enhancement and inhibition. ATF-2 = activating transcription factor-2; CB2R = cannabinoid 2 receptor; CORT = corticosterone; COX-2 = cyclooxygenase-2; EAA = excitatory amino acid; GABA = γ-aminobutyric acid; ICAM-1 = intracellular adhesion molecule-1; IL-6 = interleukin-6; IL-1β = interleukin-1β; LC = locus coeruleus; NE = norepinephrine; NMDAR = N-methyl-d-aspartate receptor; N/OFQ = nociceptin/orphanin FQ; NRM = nucleus raphe magnus; PAG = periaqueductal grey; p-Akt = phosphorylated Akt; PGE2 = prostaglandin E2; p-GluN1 = phosphorylated GluN1; SP = substance P; TNF-α = tumor necrosis factor-α; 5-HT1AR = 5-hydroxytryptamine 1A receptors.

Electroacupuncture increases opioids at inflammatory sites via two pathways. (1) It activates sympathetic nerve fibers to enhance migration of opioid-containing cells to the site. (2) It triggers hypothalamus–pituitary–adrenal to decrease COX-2, which in turn interfere with endocannabinoid metabolism, leading to increased levels of opioids at the site. Furthermore, electroacupuncture might decrease COX-2, thus lowering PGE2 levels and alleviating pain. Electroacupuncture-upregulated endocannabinoid may directly inhibit pain because CB2 receptor activation inhibits sensory nerve activities in rat pain model.30 Although peripheral CRF and adenosine are involved in electroacupuncture action, how the modality modulates their synthesis warrants further investigation.
Overall, although the current studies show that opioid, cytokines, cannabinoids, CB2R, CRF, PGE2, and adenosine are involved in acupuncture/electroacupuncture analgesia, other peripheral bioactive chemicals and receptors such as serotonin, nerve growth factor, bradykinin, and transient receptor potential channels31 are implicated in inflammatory pain. Their involvement in electroacupuncture analgesia is yet to be investigated.
Spinal Mechanisms
Several studies demonstrate that electroacupuncture inhibits peripheral inflammation-induced Fos expression in the spinal cord.32–34 These indicate that electroacupuncture dampens the transmission of noxious inputs at the spinal level with the involvement of spinal opioids, serotonin (i.e., 5-hydroxytryptamine [5-HT]), norepinephrine, glutamate, glial cell/cytokines, and signal molecules.
Opioids.
Electroacupuncture acts on spinal opioid receptors differently in acute pain and persistent inflammatory con ditions. In studies with uninjured rats, low-frequency (2 Hz) electroacupuncture at acupoint ST36 activated endorphin/endomorphin and enkephalin, whereas high-frequency (100 Hz) electroacupuncture activated dynorphin to suppress nociception as assessed by the tail flick test.35
In a systematic evaluation of the effect of electroacupuncture frequency, intensity, treatment duration, and pulse width in a CFA-induced inflammatory pain rat model, Dr. Lao et al.34 found that 10 and 100 Hz electroacupuncture at GB30 both significantly increased PWL. However, the effects of treatment at 10 Hz endured longer than did those of 100 Hz due to the greater inhibition of inflammation of the former.36 That team also showed that both 10 and 100 Hz electroacupuncture-produced inhibition of thermal hyperalgesia was blocked by intrathecal μ- and δ-opioid receptor antagonists but not a κ-opioid receptor antagonist.37 Kim et al.,38 in a capsaicin-induced inflammatory hind paw pain model, delivered 2 Hz of four-train pulses with 100 Hz of intratrain frequency at Houxi (SI3) and Sanyangluo (TE8) on the forelimb. This stimulation significantly raised the mechanical pain threshold of the injected paw, an effect blocked by intrathecal μ- or δ-opioid receptor antagonists but, again, not by a κ-opioid receptor antagonist.
In a rat model produced by a carrageenan injection into the knee cavity, pretreatment with 10 Hz electroacupuncture at ST36 before the injection significantly improved weight-bearing force, an indication of spontaneous pain, in the injected hind limb.39 This analgesic effect was blocked by an intrathecal μ-opioid receptor antagonist but not by δ- or κ-opioid receptor antagonists.39
In a study with a rat model of ankle sprain pain, 2 Hz of four-train pulses with 100 Hz of intratrain frequency was delivered on the forelimb at Yanglao (SI6) and Hegu (LI4).40 Electroacupuncture increased stepping force of the affected limb, and this analgesic effect was not blocked by systemic injection of the opioid antagonists naloxone or naltrexone. By using the same model, 10 Hz electroacupuncture at acupoint SI6 on the forelimb contralateral to the sprained ankle significantly improved stepping force of the sprained paw and suppressed spinal dorsal horn neural activity.41 This improvement in stepping force of the affected paw and electroacupuncture inhibition of spinal dorsal horn neural hyperactivity were not blocked by a 10 mg/kg intraperitoneal injection of the opioid receptor antagonist naltrexone, which implies that endogenous opioids are not involved in electroacupuncture-produced analgesia in walking-evoked pain in an ankle sprain pain model. However, this phenomenon needs to be confirmed with varying dosages of opioid receptor antagonist administered to the spinal cord, because different dosages of naltrexone produce distinct effects42 and systemic naltrexone-produced supraspinal and spinal effects might offset each other.
Collectively, these studies show that low- and high-frequency electroacupuncture, respectively, inhibit thermal pain through μ-, δ-, and κ-opioid receptors during acute pain, but during persistent pain conditions both high and low frequencies inhibit thermal, mechanical, and spontaneous pain through μ- and δ-opioid receptors (table 2). The differential involvement of opioid receptors might be due to changes in sensitivity of the receptors during persistent pain. For example, dosage–response curves for intrathecally administered μ- and/or δ-opioid agonists, determined by PWL to noxious thermal stimuli, were shifted to the left for carrageenan-inflamed hind paws in comparison with contralateral uninflamed paws. A selective intrathecal κ-receptor agonist showed no activity in this analgesic assay on either inflamed or noninflamed paws.43 μ- and δ- but not κ-receptor agonists dosage-dependently reduced mechanical hyperalgesia after repeated intramuscular injections of acid.44 Because μ-and δ-receptors are distributed on both presynaptic primary afferent fibers and postsynaptic dorsal horn neurons,45 electroacupuncture-induced opioids might inhibit the activity of noxious neurons via pre- and postsynaptic mechanisms.
The fact that electroacupuncture induces endogenous opioids to inhibit pain has clinical significance. Electroacupuncture, added to opioid therapy, might decrease the dosages required for pain control. Indeed, in our study, electroacupuncture combined with low-dosage morphine suppressed inflammatory pain better than either one did individually.37
Nociceptin/orphanin FQ (N/OFQ) and opioid-like receptors play important roles in pain modulation.46 Fu et al.47 report that 2 and 60 Hz electroacupuncture at GB30 and GB34 significantly increased N/OFQ and opioid-like receptors in the spinal cord in a CFA-induced inflammatory pain model. Moreover, intrathecal [Nphe(1)]nociceptin(1 to 13)NH(2), a selective antagonist of the N/OFQ peptide receptor, significantly blocked electroacupuncture inhibition of thermal hyperalgesia, which indicates that the N/OFQ receptor mediates electroacupuncture analgesia.48 N/OFQ has been observed in fibers and neurons in superficial laminae of the dorsal horn,49 and it inhibits C-fiber–evoked responses and wind-up.50 This leads to the conclusion that electroacupuncture attenuates pain by inducing release of spinal N/OFQ through both pre- and postsynaptic mechanisms.
Cholecystokinin octapeptide (CCK-8), a physiological antagonist of endogenous opioids in the central nervous system, attenuates electroacupuncture analgesia. Studies in uninjured animals show that electroacupuncture produces greater analgesia in CCK-8– and CCKa receptor–deficient rats than in control animals.51,52 Intracerebroventricular antisense CCK expression vector pSV2-CCKAS converts rats with low response to electroacupuncture and morphine into high responders,53 whereas subcutaneous CCK(B) receptor antagonist L365,260 produces dosage-dependent (0.125 to 2.0 mg/kg) potentiation of analgesia induced by 100 Hz electroacupuncture.54
Other studies show that CCKa receptors in the hypothalamus and nucleus parafascicularis and CCKb receptors in the periaqueductal grey (PAG) are associated with electroacupuncture analgesia.55–57 Interestingly, CCKa receptor–deficient rats with neuropathic pain showed more robust response to electroacupuncture treatment than did normal rats.58 The data lead us to hypothesize that dampened CCK function promotes acupuncture/electroacupuncture and morphine analgesia and that patients given a CCK receptor antagonist will be more sensitive to acupuncture treatment than those not given the antagonist. This warrants clinical investigation.
Norepinephrine and Serotonin.
Li et al.59 report that electroacupuncture activated serotonin-containing nucleus raphe magnus neurons and norepinephrine-containing locus coeruleus neurons that project to the spinal cord. That study and studies in uninjured rats60–63 indicate that spinal serotonin and norepinephrine are involved in electroacupuncture analgesia.
Numerous studies show involvement of serotonin and norepinephrine in electroacupuncture’s inhibition of pain. Silva et al.64 used the tail flick test in uninjured rats to show that the nonselective serotonin receptor antagonist methysergide blocked both 2 and 100 Hz electroacupuncture-produced analgesia, whereas α1- and α2-adrenoceptors antagonists only prevented 2 Hz-induced analgesia. In an intraknee carageenan-produced rat model, systemic administration of the nonselective adrenergic receptor antagonist yohimbine and the 5-HT3 receptor (5-HT3R) antagonist ondansetron blocked 2-Hz electroacupuncture-produced increase of weight bearing in an injured hind limb.65 In a collagen-induced arthritis model, systemic administration of spiroxatrine, a 5-HT1A receptor (5-HT1AR) antagonist and a 5-HT3R antagonist, blocked 2 Hz electroacupuncture analgesia evaluated with the tail flick test; ketanserin, a 5-HT2 receptor (5-HT2R) antagonist, did not.66 In a behavioral and electrophysiological study in an ankle sprain rat model, 10 Hz electroacupuncture applied contralaterally to SI6 significantly improved weight-bearing force of the sprained side and suppressed spinal dorsal horn neuron activities.41 This intervention had no effect in normal rats, and the electroacupuncture effect was blocked by the α-adrenoceptor antagonist phentolamine. These studies clearly demonstrated that serotonin and norepinephrine are involved in electroacupuncture-produced pain inhibition but did not differentiate between spinal and supraspinal levels of antagonist action.
Administration of intrathecal antagonist demonstrated that spinal norepinephrine and serotonin are involved in electroacupuncture inhibition of pain. Intrathecal yohimbine, an α2 adrenergic antagonist, reduced 2-Hz electroacupuncture-induced analgesia in a rat model of ankle sprain, but terazosin, an α1-adrenergic antagonist, had no effect.67 Consistent with these results, studies show that an intrathecal α2a-adrenoceptor antagonist blocked 10-Hz electroacupuncture antihyperalgesia in a CFA-induced inflammatory pain model, whereas an α2b-adrenoceptor antagonist did not.68 These studies indicate that spinal α2a-adrenoceptors are involved in 2 to 10 Hz electroacupuncture analgesia in inflammatory pain. In previous studies, α2a-adrenoceptor activation diminished glutamate release from the spinal cord,69 and group I metabotropic glutamate receptors enhanced phosphorylation of N-methyl-d-aspartate receptor (NMDAR) subunit GluN1 (NR1),70 which modulates NMDAR activity and promotes transmission of nociceptive inputs in inflammatory pain models.71 Moreover, intrathecal clonidine, an α2-adrenoceptor agonist, significantly suppressed GluN1 phosphorylation in a pain model.72 Immunohistochemistry shows that α2a-adrenoceptors are located in primary afferents in the spinal cord.68,73 Collectively, these studies show that electroacupuncture increases spinal norepinephrine to presynaptically decrease glutamate release, thus inhibiting GluN1 phosphorylation and pain (fig. 3).
Studies also demonstrate that 10 Hz electroacupuncture inhibits thermal hyperalgesia through spinal 5-HT1AR but not 5-HT2BR, 5-HT2CR, or 5-HT3R in a CFA-inflammatory pain model,68,74 and improves weight-bearing force through 5-HT2AR in a knee osteoarthritis pain model.75 Investigation shows that 5-HT1AR activation prevents NMDAR GluN1 subunit phosphorylation76 and that serotonin depletion increases nociception-evoked trigeminal NMDAR phosphorylation.77 Immunohistochemistry shows that 5-HT1ARs are localized in GluN1-containing neurons in the spinal cord.74 In our studies, 10 Hz electroacupuncture at GB30 inhibited CFA-upregulated p-GluN1.78 Thus electroacupuncture inhibits pain by promoting spinal 5-HT1AR activation to postsynaptically suppress GluN1 phosphorylation (fig. 3).
It is known that pretreatment with μ-receptor antagonist blocks intrathecal serotonin or clonidine-produced inhibition of inflammation-caused hyperalgesia.68 Naloxone inhibits intrathecal 5-HT antinociception,79 and selective norepinephrine reuptake inhibitors significantly increase intensity and duration of morphine antinociceptive activity via both α2 adrenergic and opioid receptors.80 Isobolographic analysis revealed a synergistic interaction between an α2-adrenoceptor agonist and morphine.81 Taken together, these data show that spinal 5-HT, norepinephrine, and opioids work in concert in electroacupuncture action. However, the mechanisms of their interaction are not clear.
Clinically, serotonin and norepinephrine reuptake inhibitors and selective serotonin reuptake inhibitors are used to manage chronic pain conditions. Because electroacupuncture induces the release of spinal 5-HT and norepinephrine, electroacupuncture treatment might enhance the inhibitory effect of serotonin and norepinephrine reuptake inhibitors/selective serotonin reuptake inhibitors on pain, thus allowing pain control medication to be decreased. This is supported by a study showing that when patients were given acupuncture/electroacupuncture as an adjunct to paroxetine, an selective serotonin reuptake inhibitor, only 5.7 to 8.9% required a dosage increase, significantly fewer than did those given paroxetine alone (22.9%).82
Glutamate and Its Receptors.
Glutamate and its receptors, categorized as NMDA, α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid, kainate, and metabotropic groups, are abundant in the spinal dorsal horn and play important roles in transmission of noxious messages.83,84 In a CFA-induced pain model, studies show that electroacupuncture at 2, 15, or 120 HZ not only significantly alleviates CFA-induced mechanical hyperalgesia but also significantly decreases CFA-upregulated GluN1, GluN2A, and GluA1 in the spinal dorsal horn.85 In dorsal root ganglia (DRG), alternation of 4 and 16 Hz electroacupuncture at GB30 and GB34 significantly inhibited CFA-induced GluN1 up-regulation.86 In our studies, 10 Hz electroacupuncture at GB30 significantly decreased CFA-enhanced GluN1 phosphorylation.78 Collectively, these studies demonstrate that electroacupuncture inhibits transmission of noxious messages at the spinal level by dampening glutamate receptor activities.
Electroacupuncture might work through spinal norepinephrine, 5-HT, and opioids to decrease NMDAR activation and thus to inhibit pain. As mentioned in section Norepinephrine and Serotonin, electroacupuncture might inhibit GluN1 phosphorylation by inducing spinal release of 5-HT and norepinephrine. It has been reported that microiontophoretic application of a selective μ-opioid receptor agonist, [d-Ala2,N-Me-Phe4,Gly-ol5]enkephalin, reduced the NMDA-evoked responses of 100% of nociceptive specific, 93% of wide dynamic range, and 86% of low-threshold neurons in the superficial and deeper dorsal horn of the medulla.87 These studies show that electroacupuncture-induced endogenous opioids inhibit NMDA-mediated excitation in the spinal cord, thus alleviating pain. Hence, electroacupuncture inhibits NMDAR by activating at least three pathways, norepinephrine, 5-HT, and opioid, thus resulting in the alleviation of pain (fig. 3).
As in the electroacupuncture plus morphine study,37 electroacupuncture with low-dosage dizocilpine maleate (MK-801; a noncompetitive NMDAR antagonist) suppressed inflammatory pain better than did either modality alone.88,89 The data from these studies once again provide a rationale for pain management and control that combine Western medicine and acupuncture, and they corroborate previously reported data: for example, etoricoxib plus acupuncture has been shown to be more effective than etoricoxib with sham acupuncture or etoricoxib alone for the treatment of knee osteoarthritis pain.90
Glial Cells/Cytokines.
Electroacupuncture inhibits spinal glial cell activation and decreases cytokines to alleviate pain. Alternating 2 and 100 Hz at GB30 and GB34 raised PWL and markedly inhibited intraarticular CFA-induced astrocyte and microglia activation and IL-1β, IL-6, and TNF-α up-regulation in the spinal cord.91–93 It is well known that spinal proinflammatory cytokines facilitate pain. For example, IL-1β enhances GluN1 phosphorylation to facilitate pain,94–96 and TNF-α increases NMDA currents in spinal lamina II neurons.97 Thus, electroacupuncture might inhibit glial cell activation to decrease production of proinflammatory cytokines, leading to the inhibition of pain. Moreover, 2 Hz electroacupuncture at ST36 and Sanyinjiao (SP6) once a day for 3 days significantly increased PWL and inhibited CFA-induced up-regulation of glial fibrillary acidic protein, an astrocyte marker.98 It also reversed CFA-caused down-regulation of glutamate–aspartate transporter and glutamate transporter-1 in astrocytes. Concomitantly, proteasome inhibitor MG132 treatment significantly increased PWL and reversed CFA-induced down-regulation of glutamate–aspartate transporter and glutamate transporter-1. Because electroacupuncture decreased CFA-enhanced spinal proteasome activity, it is concluded that electroacupuncture inhibition of proteasome restores glutamate transporters, leading to pain inhibition.98
Opioid and N/OFQ peptide receptor activation might mediate electroacupuncture inhibition of cytokine synthesis in the spinal cord (fig. 3). Substance P (SP), a neuropeptide key to the transmission of noxious inputs, activates glial cells during pain.99 Its release is blocked by μ- and δ-opioid receptors,100 which electroacupuncture significantly activates.37 Electroacupuncture also inhibits tooth pulp stimulation–evoked increase in the release of immunoreactive SP.101 These data indicate that electroacupuncture-induced endogenous spinal opioids decrease the release of neurotransmitters such as SP and inhibit cytokine synthesis in glial cells.
It has been reported that intrathecal administration of N/OFQ significantly down-regulates CFA-exaggerated IL-1β, IL-6, and TNF-α mRNA in the spinal cord in the inflammatory pain rat model, actions which were abolished when combined with the Opiate receptor-like 1-receptor–specific antagonist [Nphe(1)]N/OFQ(1–13)NH2.102 Opiate receptor-like 1-receptors are expressed on astrocytes in the rat spinal cord. In vitro studies with astrocyte cultures also show that N/OFQ inhibits cytokine gene expression through the Opiate receptor-like 1-receptor.102 Because electroacupuncture induces release of spinal N/OFQ,47,48 it is plausible that electroacupuncture-induced spinal N/OFQ suppresses spinal cytokine synthesis (fig. 3).
Signal Molecules.
The influence of acupuncture on signal molecules and signal pathways has been investigated. In a carrageenan-induced inflammatory pain model, 2 Hz electroacupuncture at ST36 and SP6 significantly decreased mechanical and thermal hypersensitivity and simultaneously inhibited carrageenan-induced phosphorylation of phosphatidylinositol-3-kinase and Akt.103 It has been reported that SP enhances Akt phosphorylation in neurokinin-1 receptor–positive neurons in laminae I to III and that phosphorylated Akt initiates and maintains inflammatory hyperalgesia.104 It is well known that activation of spinal μ- and δ-opioid receptors potently inhibits the SP release induced by peripheral noxious stimuli.100 These studies show that electroacupuncture-induced endogenous opioids inhibit SP release and Akt phosphorylation to alleviate pain (fig. 3). Moreover, in a study by Liu et al.,105 10-Hz electroacupuncture-produced inhibition of inflammatory thermal hyperalgesia was prevented when spinal Gi/o-protein function was destroyed by intrathecal pretreatment with pertussis toxin. This indicates that electroacupuncture-produced antihyperalgesia is mediated by pertussis toxin–sensitive Gi/o proteins and the relevant signaling pathways.
Alternating 2 and 100 Hz electroacupuncture bilaterally at ST36 and Kunlun (BL60) inhibited and decreased CFA-induced pain, p38 mitogen–activated protein kinase (MAPK), and activating transcription factor-2–positive cells in the dorsal spinal cord.106,107 Activated p38 MAPK is known to play an important role during nociception and is translocated to the nucleus to phosphorylate transcriptional factors such as activating transcription factor-2.108 Neurokinin-1 receptors and proinflammatory cytokines such as IL-1β and TNF-α contribute to p38 MAPK activation in the spinal cord.108,109 Conclusively, electroacupuncture-induced inhibition of proinflammatory cytokines as detailed in section Glial Cells/Cytokines and electroacupuncture inhibition of neurokinin-1 receptors110 inhibit p38 MAPK/activating transcription factor-2 to suppress pain (fig. 3).
Electroacupuncture at 2 Hz at ST36 inhibited intraplantar carrageenan- and CFA-induced up-regulation of acid-sensing ion channel 3 in DRG.111 At present, it is unknown how electroacupuncture modulates this channel and the relevant signal cascades.
Other Substances.
Although studies in uninjured animals have demonstrated the involvement of muscarinic, γ-aminobutyric acid (GABA), and dopamine receptors in electroacupuncture analgesia,6060,64,112112 few studies have used persistent pain animal models. In an intraknee carrageenan rat model, systemic administration of the dopamine D2 receptor antagonist metoclopropamide blocked electroacupuncture analgesia.65 This is consistent with dopamine D2 agonist inhibition of pain.113 Furthermore, dopamine D2 agonists enhance the antinociception of 5-HT and norepinephrine reuptake inhibitors.113 Electroacupuncture-induced dopamine, 5-HT, and norepinephrine might alleviate pain synergistically. In collagen-induced arthritis, systemic administration of atropine, a muscarinic cholinergic receptor antagonist, also blocked electroacupuncture analgesia.66 Because spinal muscarinic receptors are involved in morphine- and clonidine-produced antinociceptive effects,114,115 electroacupuncture-induced acetylcholine, opioids, and norepinephrine also might alleviate pain synergistically.
Spinal mechanisms of electroacupuncture on inflammatory pain have been extensively investigated with behavioral, molecular, and pharmacological approaches. Electroacupuncture might induce several neurotransmitters, including opioids, 5-HT, norepinephrine, dopamine, and acetylcholine, which work interactively to inhibit pain. It is unclear how electroacupuncture induces these neurotransmitters.
Supraspinal Mechanisms
Electroacupuncture Inhibition of the Sensory Dimension of Pain.
Zhao’s60 2008 review summarizes a number of studies in uninjured animals. Collectively, these show that many nuclei, including the nucleus raphe magnus, PAG, locus coeruleus, arcuate, preoptic area, nucleus submedius, habenular, accumbens, caudate, septal area, and amygdala, are involved in acupuncture analgesia and that opioid peptides and their receptors in the arcuate-PAG-nucleus raphe magnus-spinal dorsal horn pathway are pivotal in this effect.60,116 Recently, brain mechanisms of electroacupuncture analgesia have been investigated in an inflammatory pain model. Selective μ- but not κ-receptor antagonists infused into the rostral ventromedial medulla (RVM) blocked 10 and 100 Hz electroacupuncture-produced antihyperalgesia assessed with PWL.117 Double immunofluorescence staining demonstrated that μ-receptor–containing neurons are GABAnergic and that GABAa receptor–containing neurons are serotonergic in the RVM.117 Hence, electroacupuncture induces release of endogenous endomorphins. These activate μ-opioid receptors in GABAnergic neurons that suppress GABA release. Without GABA inhibition, RVM-spinal cord projecting serotonergic neurons can be activated to inhibit inflammatory pain.59
In a carrageenan model of inflammatory pain, 4 and 60 Hz electroacupuncture alternated at ST36 and BL60 significantly inhibited thermal hyperalgesia and carrageenan-enhanced IL-1 receptor type I mRNA expression in the PAG compared with sham control.118 As μ-opioids and chemokine receptors interact,119 electroacupuncture-induced opioids might act on the latter to inhibit IL-1 receptors. In addition, it has been reported that melanocortin 4 receptor blockage in the PAG significantly attenuated mechanical allodynia and thermal hyperalgesia and inhibited glial activation and IL-1β synthesis.120 It is possible that electroacupuncture inhibits melanocortin 4 receptors to attenuate IL-1β and IL-1 receptor activity in the PAG, thus alleviating pain.
Compared with the spinal level, brain involvement in electroacupuncture analgesia during persistent pain has been less investigated. As pain sensation is dependent on brain function and subject to brain modulation, electroacupuncture modulation of brain might play an important role in pain inhibition. For example, deep brain stimulation of the ACC in humans significantly inhibited pain.121 Brain mechanisms of electroacupuncture analgesia warrant further investigation.
Electroacupuncture Inhibition of the Affective Dimension of Pain.
Pain has two dimensions, the sensory/discriminative and the affective. Studies in animal pain models show that electroacupuncture inhibits the sensory dimension of pain by producing an antinociceptive effect; electroacupuncture action on the affective component has only recently been studied. A CFA-inflammatory pain rat model was combined with a conditioned place avoidance test to determine whether electroacupuncture inhibits pain-induced affective response.122 During preconditioning, rats spent similar amounts of time in two compartments, indicating no aversion to either. After conditioning, rats that received sham electroacupuncture spent less time in a pain-paired compartment, demonstrating place aversion to that compartment. By contrast, electroacupuncture-treated rats showed no aversion to the pain-paired compartment, demonstrating that electroacupuncture inhibited the CFA-produced affective response.122 Saline-injected rats showed neither preference nor aversion to electroacupuncture- or sham electroacupuncture–paired chambers; this shows that the electroacupuncture treatment did not produce reward or aversion (fig. 4). Intrarostral ACC (rACC) pretreatment with a μ- but not a κ-opioid receptor antagonist blocked electroacupuncture inhibition of pain-related affective response.122 A microinjection of morphine into the ACC inhibited affective response but did not change the sensory pain threshold,123 and an intrathecal μ-opioid receptor antagonist blocked electroacupuncture-produced increase of PWL but not electroacupuncture inhibition of affective response.122 Together, these studies show that electroacupuncture induces a release of endorphins to block affective response and that this effect is not a consequence of sensory pain inhibition. Interestingly, animal studies demonstrate that several drugs, including morphine, oxycodone, tramadol, ibuprofen, and pregabalin, show a clear dissociation between antiaversive and antinociceptive potency.124 Clinical studies found that morphine more potently attenuates the affective component than the sensory component of pain.125 Thus electroacupuncture also might differ in its actions on the sensory and affective dimensions of pain.
Fig. 4.
Electroacupuncture inhibited the affective component of pain. Conditioned place avoidance scores, used to indicate affective response, were determined by subtracting time spent in the pain-paired compartment during preconditioning from time spent in that compartment during the postconditioning test: the less postconditioning time spent in the compartment, the greater the affective response. *P < 0.05 compared with sham control in complete Fruend’s adjuvant (CFA)–injected rats. CPA = conditioned place avoidance; EA = electroacupuncture. Reprinted with permission from Elsevier, Eur J Pain 16(2), 2012.

In another study, electroacupuncture attenuated formalin-induced conditioned place avoidance.126 Furthermore, selectively blocking the GluN2A or GluN2B subunit of the rACC abolished intraplantar formalin injection–induced affective pain but not the nociceptive behaviors.127 It is known that the μ-opioid receptor agonist [d-Ala2,N-Me-Phe4,Gly-ol5]enkephalin significantly decreases both NMDA and non-NMDA excitatory postsynaptic potential amplitudes in nucleus accumbens neurons; this action is reversed by a μ-opioid receptor antagonist.128 Immunohistochemical data showed that NMDAR and μ-receptors are colocated in rACC neurons (fig. 5). The hypothesis is that electroacupuncture-produced μ-opioid receptor activation modulates NMDAR activities to attenuate pain-associated affective response (fig. 3).
Fig. 5.
Microphotographs showing distribution and colocalization of GluN1 and μ-opioid receptors in rostral anterior cingulate cortex neurons. (A and B) Sections were double-labeled with guinea pig polyclonal antibody against μ-opioid receptors (A, red) and goat polyclonal antibody against GluN1 (B, green). (C) Merged A and B photographs showing colocalization of μ-opioid receptors and GluN1 in the rostral anterior cingulate cortex. Arrows point to double-labeled neurons (yellow). Scale bar = 50 μm.

In summary, electroacupuncture inhibits both sensory and affective dimensions of pain. Electroacupuncture-induced endogenous opioids in the rACC may suppress NMDAR functions, which play an important role in electroacupuncture inhibition of the affective dimension of pain. It is unclear whether other neurotransmitters, such as norepinephrine which is located in the ACC, are involved in electroacupuncture inhibition of the affective component of pain.
Neuropathic Pain Models
Although mechanisms of acupuncture analgesia have been investigated at each level of the pain pathway in inflammatory pain models, acupuncture analgesia has been studied mainly at the spinal level in neuropathic pain models129 and shows involvement of opioids, serotonin, norepinephrine, amino acids, and glia cell/cytokines.
Opioids
Spinal opioids are involved in electroacupuncture inhibition of neuropathic pain. Systemic administration of naloxone blocked 2 Hz electroacupuncture inhibition of L5 and L6 spinal nerve ligation (SNL)– and caudal trunk nerve injury–induced neuropathic pain.130,131 Manual acupuncture, applied at ST36 and SP6 in an SNL-induced neuropathic pain rat model, significantly reduced SNL-induced hypersensitivity; this effect was blocked by systemic nolaxone.132 Furthermore, administration of cumulative doses (5, 10, and 20 nmol) of the μ- or δ-opioid receptor antagonists β-funaltrexamine hydrochloride and naltrindole blocked 2-Hz–produced electroacupuncture antimechanical allodynia, corroborating μ- and δ-opioid receptor involvement in electroacupuncture action. Cumulative doses of the κ-opioid receptor antagonist nor-binaltorphimine at 3, 6, and 12 nmol did not significantly influenced electroacupuncture inhibition of pain,133 possibly due to the low dosages administered. Moreover, chemotherapy-induced peripheral neuropathy is the most common and serious adverse effect of chemotherapeutic agents.134 A paclitaxel-evoked peripheral neuropathy model has been developed using intraperitoneal 2 mg kg−1 ml−1 of paclitaxel on 4 alternate days (0, 2, 4, and 6).135 Beginning on day 13, the response frequency to von Frey filaments (bending force: 4 to 15 g) was significantly increased in paclitaxel-injected rats compared with that in those injected with vehicle. Electroacupuncture at 10 Hz at GB30 significantly decreased response frequency at 4 to 15 g compared with sham electroacupuncture136 ; 10 Hz electroacupuncture plus a μ-, δ-, or κ-opioid receptor antagonist did not significantly decrease mechanical hypersensitivity compared with sham electroacupuncture plus vehicle; this shows that all three antagonists blocked electroacupuncture action in this model. Clearly, μ-, δ-, and κ-receptors are involved in 10 Hz electroacupuncture inhibition of chemotherapy-induced neuropathic pain; this is different from the opioid involvement in electroacupuncture inhibition of peripheral tissue injury–caused inflammatory pain, in which only μ- and δ-receptors participate (table 2). It has been reported that an interaction exists between μ- and κ-receptors: spinal μ-receptor activation might promote κ-receptor activation–produced antinociception.137 Thus it is hypothesized μ- and κ-receptor interaction is modulated differently in neuropathic and inflammatory pain conditions. This would account for the differential involvement of opioid receptors in electroacupuncture action.
Low-frequency electroacupuncture inhibits neuropathic pain more effectively than does high-frequency electroacupuncture. Electroacupuncture at 10 Hz at GB30 significantly decreased mechanical response frequency at 4 to 15 g compared with sham electroacupuncture136 ; 100 Hz electroacupuncture only decreased response frequency at 15 g in a paclitaxel-evoked peripheral neuropathy model. This indicates that 10 Hz electroacupuncture inhibits mechanical allodynia/hyperalgesia more potently than 100 Hz does.136 Similarly, 2 Hz electroacupuncture, in a caudal trunk nerve injury–induced neuropathic pain model, induced a robust and longer-lasting inhibitory effect on mechanical allodynia assessed with a von Frey filament (bending force: 2 g) than did 100 Hz.133 Consistent with these results, 2 Hz decreased mechanical and thermal hypersensitivity more powerfully in an L5/L6 nerve ligation–induced neuropathic pain model than did 100 Hz.138 A single treatment at 2 Hz electroacupuncture inhibited thermal hypersensitivity for 48 h; a single treatment at 100 Hz electroacupuncture was only effective for 8 h.138 In an electrophysiological study,139 2 Hz electroacupuncture induced long-term depression of C-fiber–evoked potentials in rats with SNL, whereas 100 Hz electroacupuncture induced long-term potential in SNL rats. Synaptic long-term depression in the spinal dorsal horn in SNL rats might be involved in the long-lasting analgesia produced by 2 HZ electroacupuncture.
Serotonin and Norepinephrine
Spinal serotonin and norepinephrine participate in electroacupuncture inhibition of neuropathic pain. For example, 2 Hz electroacupuncture inhibited cold allodynia in rats with right caudal trunk resection between the S1 and S2 spinal nerves innervating the tail.140 Intrathecal injection of the 5-HT1AR antagonist NAN-190 and the 5-HT3R antagonist MDL-72222 significantly blocked this effect; the 5-HT2AR antagonist ketanserin did not. Intrathecal injection of the α2-adrenoceptor antagonist yohimbine also significantly blocked electroacupuncture action, but the α1-adrenoceptor antagonist prazosin did not. These data show that spinal α2-adrenoceptors, 5-HT1ARs, and 5-HT3Rs are involved in electroacupuncture inhibition of cold allodynia in neuropathic pain. Although α2-adrenoceptors and 5-HT1ARs are involved in electroacupuncture inhibition of both inflammatory and neuropathic pain, 5-HT3Rs play a role in electroacupuncture inhibition of neuropathic but not inflammatory pain. Consistent with that activity, 5-HT3Rs are involved in spinal cord stimulation-induced analgesia during neuropathic pain.141 It is possible that nerve injury changes spinal 5-HT3R activities leading to the receptors’ participation in neuropathic pain modulation.
It has been reported that 2 Hz electroacupuncture inhibits nerve injury–induced GluN1 expression in the spinal cord131 and that 5-HT1ARs are located on GluN1-containing spinal cord neurons.68 These data lead us to hypothesize that electroacupuncture activates 5-HT1ARs to inhibit NMDAR activities, thus attenuating neuropathic pain.
Although inflammatory and neuropathic pain differ, certain central spinal and brain mechanisms such as activation of NMDA receptors are common to both.142 Interestingly, electroacupuncture attenuates both inflammatory and neuropathic pain by activating spinal α2-adrenoceptors and 5-HT1ARs and inhibiting GluN1 (fig. 3).
Amino Acids
Both excitatory amino acids and inhibitory amino acids play roles in electroacupuncture attenuation of neuropathic pain. In a study with a chronic constriction injury model, 2 Hz electroacupuncture significantly increased thermal and mechanical threshold, decreased concentration of spinal glutamate, aspartate, and glutamine, and increased the contents of glycine, GABA, and taurine in the spinal cord.143 Because this study did not use a sham electroacupuncture control, those data need to be confirmed. Furthermore, microdialysis was used to collect the dialysate from the spinal cord in a spared nerve injury rat model. Electroacupuncture significantly decreased dialysate glutamate compared with sham electroacupuncture.144 Moreover, intrathecal administration of the GABA(A) or GABA(B) receptor antagonists gabazine and saclofen blocked electroacupuncture inhibition of cold allodynia in the rats with resected caudal trunk nerves.145 Collectively, these studies demonstrate that electroacupuncture reduces the release of excitatory amino acids and promotes the release of inhibitory amino acid neurotransmitters to stop pain. With regard to excitatory amino acids, morphine decreased the formalin-enhanced release of glutamate in the spinal dorsal horn,146 presynaptic 5-HT1BRs decreased glutamate release from primary afferent terminals onto medullary dorsal horn neurons,147 and α2a-adrenoceptor activation diminished glutamate release from the spinal cord in previous studies.69 On the basis of those studies, it is proposed that endogenous electroacupuncture-induced opioids, 5-HT, and norepinephrine suppress excitatory amino acid release (fig. 3). Regarding the inhibitory amino acid GABA, a study showed that 5-HT1ARs are expressed on GABAergic neurons.148 It seems that electroacupuncture-induced 5-HT promotes the inhibitory effect of GABA to inhibit pain. Moreover, activation of δ-opioid receptors reduces GABA uptake149 ; thus electroacupuncture-induced endogenous opioids might increase GABA in extracellular space, enhancing GABA function and leading to pain inhibition (fig. 3). In addition, intrathecal administration of the GABA receptor A or B agonists muscimol and baclofen potentiated the antinociceptive effects of morphine.150 Thus it is plausible that endogenous opioids and GABA interact during electroacupuncture treatment to alleviate pain.
Glial Cells/Cytokines
Glial cell and cytokines participate in electroacupuncture inhibition of neuropathic pain. For instance, 2 Hz electroacupuncture at ST36 significantly increased mechanical and thermal thresholds in rats with superior caudal trunk transection; it significantly inhibited nerve-damage–induced up-regulation of glial fibrillary acidic protein and OX-42, matrix metallopeptidase-9 and matrix metallopeptidase-2, TNF-α, IL-6, and IL-1β in the spinal cord.151 In a spinal cord injury rat model, manual acupuncture at GB34 and Shuigou (DU26) significantly relieved mechanical allodynia and thermal hyperalgesia.152 Acupuncture also inhibited microglia activation, p38 MAPK and extracellular signal-regulated kinase phosphorylation, and superoxide anion production in microglia; it decreased IL-1β, IL-6, TNF-α, inducible nitric oxide synthases, COX-2, and PGE2 levels in the lumbar4-5 spinal cord.152 In rats with tightly ligated and transected tibial and sural nerves, 1 Hz electroacupuncture at ST36 and Yinlingquan (SP9) significantly decreased nerve injury-enhanced immune response expression of IL-1β, IL-6, and TNF-α in peripheral nerves.153 Electroacupuncture also inhibited spinal Cox-2 in the SNL model.154 These chemicals positively promote transmission of noxious inputs at the spinal level; their inhibition might contribute to electroacupuncture analgesia. As stated in section Glial Cells/Cytokines under inflammatory pain models, activation of opioid and N/OFQ peptide receptor might mediate electroacupuncture inhibition of cytokine synthesis in the spinal cord. In addition, p38 MAPK and extracellular signal-regulated kinase phosphorylation mediate the development of tolerance to morphine-induced analgesia,155 making electroacupuncture inhibition of this phosphorylation clinically relevant for the purpose of mitigating or delaying the development of morphine tolerance.
Other Bioactive Molecules
Muscarinic receptors and glial cell line–derived neurotrophic factor (GDNF) are involved in electroacupuncture action. One study demonstrates that spinal muscarinic M(1) subtype receptors mediate electroacupuncture-induced antiallodynia in neuropathic rats.156 This is consistent with a report that M1 receptor activation induced analgesia in a neuropathic pain model.157 Electroacupuncture significantly enhanced expression of somatostatin, GDNF, and the GDNF family receptor GFRα-1 (the high-affinity receptor of GDNF) in DRG and the spinal dorsal horn in rats with neuropathic pain.158,159 That pretreatment with antisense oligodeoxynucleotide, specifically against GFRα-1 attenuates electroacupuncture analgesia, indicates that electroacupuncture enhancement of GFRα-1 contributes to analgesia.160 This is supported by the fact that overexpression of GDNF in the uninjured DRG exerts analgesic effects on neuropathic pain.161 Interestingly, other studies showed that 5-HT induces GDNF mRNA expression in rat C6 glioma cells162 and that GDNF receptor activation significantly suppresses channel activities of transient receptor potential cation channel subfamily A, number 1 that facilitate the sensation of pain.163 We conclude that electroacupuncture-induced enhancement of serotonin activities increases GDNF synthesis, then decreases channel activities of transient receptor potential cation channel subfamily A, number 1 in the spinal cord to suppress pain.
Moreover, electroacupuncture has been shown to inhibit P2X3 to attenuate neuropathic pain.164 Electroacupuncture at ST36 induces nitric oxide in the gracile nucleus to attenuate pain in Zucker diabetic fatty rats.165 In contrast, another study showed that electroacupuncture at ST36 and SP9 inhibits nitric oxide synthase expression in the spinal cord of neuropathic rats.166 Involvement of nitric oxide in electroacupuncture analgesia needs to be confirmed.
Overall, electroacupuncture induces numerous bioactive chemicals that have inhibited neuropathic pain in several rat models (fig. 1). Whether and how it modulates supraspinal and peripheral nerve activities, which might be responsible for electroacupuncture inhibition of neuropathic pain, have not been investigated as yet.
Cancer Pain Models
Electroacupuncture has alleviated bone cancer pain in animal models. Studies in a prostate cancer pain rat model clearly show that 10 Hz electroacupuncture for 30 min a day at the equivalent of human acupoint GB 30 between days 14 and 18 after a cancer cell injection significantly attenuated both thermal and mechanical hyperalgesia.167,168 Moreover, electroacupuncture treatment inhibited up-regulation of preprodynorphin mRNA, dynorphin, and IL-1β and its mRNA compared with sham control. Intrathecal antiserum against dynorphin A (1 to 17) and an IL-1 receptor antagonist significantly suppressed cancer-induced hyperalgesia. These studies show that electroacupuncture inhibition of dynorphin and IL-1β contribute to electroacupuncture analgesia in cancer pain models.167,168
In another model, S-180 sarcoma cells were injected around the left sciatic nerve of BALB/c mice to cause mechanical allodynia.169 Nine days of 2 Hz electroacupuncture at ST36 once a day for 30 min significantly inhibited thermal hyperalgesia and spontaneous pain. Electroacupuncture also inhibited SP expression in the spinal dorsal horn and increased the concentration of β-endorphin in the blood and brains of mice.169 Those data warrant further confirmation because the study did not include a sham control.
In a third model, Walker 256 carcinoma cells were subcutaneously injected into the plantar region of the hind paw to cause pain,170 and 2 Hz electroacupuncture, administered bilaterally at ST36, significantly decreased the induced mechanical and thermal hypersensitivity and spontaneous pain. It also markedly suppressed cancer-driven up-regulation of transient receptor potential cation channel subfamily V member 1 expression in corresponding L3-5 DRG. Because transient receptor potential cation channel subfamily V member 1 facilitates cancer pain, electroacupuncture inhibition of this protein might diminish pain.171 Taken together, these findings indicate that electroacupuncture treatment significantly inhibits cancer pain. Because cancer-related pain is debilitating and has not been well controlled, electroacupuncture might be a useful adjuvant therapy in patients with such pain.
Visceral Pain Models
Increasing evidence shows that acupuncture effectively alleviates visceral pain in animal models through peripheral, spinal, and supraspinal mechanisms (fig. 6).
Fig. 6.
Mechanisms of electroacupuncture inhibition of visceral pain. The symbol “−” represents inhibition. CRF = corticotrophin-releasing factor; DRN = dorsal raphe nucleus; NK-1 = neurokinin-1; RVM = rostral ventromedial medulla; SP = substance P; TNF-α = tumor necrosis factor-α; VIP = vasoactive intestinal polypeptides; VIPR = VIP receptor; 5-HT = 5-hydroxytryptamine.

Peripheral Mechanisms
Numerous peripheral chemicals including neurotransmitters, neuropeptides, and cytokines are involved in electroacupuncture inhibition of visceral pain. Alternation of 5 and 100 Hz electroacupuncture bilaterally at ST36 significantly decreased visceral pain and colon 5-HT3R levels in an irritable bowel syndrome (IBS) model induced by intrarectal administration of acetic acid.172 Alternating 2 and 50 Hz electroacupuncture at ST25 and ST37 (Shangjuxu) significantly decreased colorectal distension–induced abdominal withdrawal reflex (AWR), the number of mucosal mast cells, SP, neurokinin-1 receptors, vasoactive intestinal polypeptides (VIPs), and VIP receptor expression in the colon in an IBS rat model induced by mechanical colorectal irritation.173,174 Electroacupuncture at ST36 significantly decreased colon damage and serum and colon TNF-α mRNA expression in rats with ulcerative colitis induced by intracolon ethanol and 2,4,6-trinitrobenzenesulfonic acid.175 These studies demonstrate that electroacupuncture relieves visceral pain by modulating mast cells, SP, VIP, 5-HT3Rs, and TNF-α function. In keeping with the electroacupuncture studies, a study demonstrated that blockage of 5-HT3Rs significantly increases colonic distension perception threshold in IBS patients with diarrhea.176 Studies also show that SP, serotonin, and histamine, which can be released by mast cells, sensitize visceral afferents.177 Patients with recurrent abdominal pain showed significantly greater expression of VIP, neurokinin-1 receptors, and TNF-α mRNA compared with control.178,179 Thus it seems that electroacupuncture decreases numerous chemicals at peripheral sites to desensitize visceral afferents and alleviate visceral pain.
Spinal Mechanisms
Opioids are involved in electroacupuncture inhibition of visceral pain. A study demonstrated that alternating 4 and 100 Hz electroacupuncture bilaterally at ST36 and ST37 significantly decreases colorectal distension–induced AWR and the magnitude of electromyograms.180 In another study, alternating 2 and 100 Hz electroacupuncture bilaterally at ST36 significantly attenuated visceral motor response to colorectal distension and the hyperexcitability of colon DRG neurons in rats instilled with intracolonic acetic acid181 ; this attenuation was prevented by intraperitoneal naloxone pretreatment, displaying the involvement of opioids without differentiation between peripheral, spinal, and supraspinal levels of opioid action. Pretreatment with 20 Hz electroacupuncture at Jiaji (EX-B2) significantly inhibited intracolonic formalin-caused visceral pain behavior, including abdominal licking, backward extension, contraction of the flanks, and whole body contraction.182 It suppressed p38 phosphorylation and Fos expression in the spinal cord and colon, indicating that electroacupuncture modulates spinal neuronal activities. It also increased serum β-endorphin, which might act on peripheral and central site to suppress pain. Those changes were not found in healthy electroacupuncture-treated rats,182 demonstrating that electroacupuncture modulates the nervous system differently in healthy animals and those with pain conditions. Indeed, it has been shown that patients with Bell’s palsy have distinct brain responses to acupuncture treatment compared with healthy volunteers.183 Furthermore, patients at different stages of Bell’s palsy varied in brain responses to acupuncture. Taken together, electroacupuncture alleviates visceral pain by modulating spinal and DRG activities through endogenous opioids. Phosphorylated p38 in microglia is known to be involved in inflammatory, neuropathic, and visceral pain.108,184 As discussed in section Glial Cells/Cytokines under inflammatory pain models, electroacupuncture-induced endogenous opioids reduce the release of neurotransmitters such as SP and subsequently inhibit glial cell activation, which might decrease p38 phosphorylation and inhibit visceral pain.
Supraspinal Mechanisms
Electroacupuncture might also attenuate visceral pain through supraspinal mechanisms. An early study showed that vasopressinergic neurons in the hypothalamic paraventricular nucleus participate in the electroacupuncture inhibition of visceral pain induced by an intraperitoneal injection of antimonium potassium tartrate.185 In a gastric distension–induced pain model, 10 or 100 Hz electroacupuncture at ST36 significantly mitigated visceral pain assessed with AWR and increased hypothalamus β-endorphin and SP.186 In an IBS rat model produced by mechanical colorectal irritation, alternating 2 and 50 Hz electroacupuncture at ST37 significantly inhibited visceral pain and hypothalamic CRF synthesis.187 In a neonatal maternal separation stress-induced IBS rat model, 10 Hz electroacupuncture at ST36 significantly suppressed visceral hyperalgesia and inhibited Fos expression in dorsal raphe nuclei of the brainstem, the superficial dorsal horn of the spinal cord, and colonic epithelium as well as 5-HT expression in dorsal raphe nuclei and the spinal cord.188
In an electrophysiological study in anesthetized healthy rats, response to colorectal distension by convergent somatic and visceral neurons in the thalamic ventrobasal nucleus was inhibited by electrical stimulation at either the skin receptive field or acupoint ST36.189 It is interesting that skin receptive fields of thalamic neurons, which respond to visceral nociception, lie more or less along traditional Chinese medicine’s Stomach Channel and that simulating the receptive field produces more robust inhibition than does stimulating ST36, an important point on that channel. However, it has not been determined whether the same phenomenon can be found for such behavioral tests as AWR. Moreover, alternating 5 and 100 Hz electroacupuncture at ST36 and ST37 decreased AWR- and IBS-exaggerated GluN1 and Fos expression in the RVM.190 Because an intra-RVM NMDAR antagonist inhibited visceral pain,191 electroacupuncture might relieve visceral pain by inhibiting NMDAR activation in the RVM.
Collectively, several chemicals, including β-endorphin and 5-HT, are involved in electroacupuncture inhibition of visceral pain (fig. 6), but the underlying mechanisms are not yet understood. Also, although brain structures such as the thalamic ventrobasal nucleus, the hypothalamus, paraventricular nucleus, dorsal raphe nuclei, and the RVM are involved in electroacupuncture action, how those structures work together to modulate visceral pain during electroacupuncture treatment warrants further investigation.
Clinical Implications
The preclinical studies summarized in this review provide solid evidence that electroacupuncture treatment, compared with sham electroacupuncture, results in significant changes in bioactive chemicals—including opioids, N/OFQ, serotonin, norepinephrine, glutamate receptors and transporters, cytokines, and signal molecules—in peripheral injury sites, the spinal cord, and supraspinal structures. Findings from animal models demonstrate that electroacupuncture and sham control work through different mechanisms, which implies that patients with persistent pain might respond differently to electroacupuncture and sham controls. Indeed, electroacupuncture led to significantly higher activation at the right insula, pulvinar, and medial nucleus of the thalamus compared with sham control in patients with IBS.192
In animal models, electroacupuncture significantly inhibits pain in comparison with sham control. Systematic reviews have shown that in clinical studies, most of which used manual acupuncture, acupuncture performed either significantly better193 or not significantly better than sham although both improved pain score.194 Interestingly, in some clinical studies, electroacupuncture produced higher pain threshold increase195 and significantly better subjective evaluations of treatment effects196 than did manual acupuncture. Furthermore, early clinical trials with electrical stimulation of acupoints in patients with osteoarthritis showed significant pain inhibition compared with sham control.2,197,198 This is confirmed by a more recent study using electrical stimulation of acupoints during treatment.90 In contrast, manual acupuncture produced no significant effects compared with sham control.199,200 On the basis of that evidence, we hypothesize that electroacupuncture is superior to manual acupuncture, but further investigation is warranted to confirm this premise.
The studies using electroacupuncture plus pain medication provide significant and useful information for maximizing the effect of integrative medicine in clinical settings.90 Such combination treatments might provide effective strategies for pain management, both by enhancing treatment effectiveness and by lowering pain medication dosages thus decreasing the risk of debilitating adverse effects. For example, in an animal study, electroacupuncture combined with a subeffective dose of morphine enhanced inflammatory pain inhibition compared with morphine.37 In a clinical study,201 electroacupuncture treatment before surgery significantly decreased the total amount of morphine required during the first 24 h postoperation and significantly reduced the incidence of nausea and dizziness during the same period compared with sham electroacupuncture. Similarly, electroacupuncture enhanced the effects of low-dosage celecoxib on monoarthritic pain202 and low-dosage indomethacin on CFA-induced inflammatory pain,203 as well as intrathecal antisense oligodeoxynucleotide to IL-1 receptor type I204 and GluA (α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid)/GluK (Kainate) receptor antagonists33 on carrageenan-induced pain. These studies show that electroacupuncture treatment has the potential to allow the use of lower dosages of such medications in clinical settings, thus reducing side effects and improving patient quality of life.
These animal studies also show that electroacupuncture inhibition of pain is parameter dependent. Electroacupuncture at 10 Hz produces longer alleviation of inflammatory pain and more powerful inhibition of neuropathic pain than does 100 Hz electroacupuncture.34 Electroacupuncture at 10 Hz inhibits inflammation, whereas electroacupuncture at 100 Hz does not.36 This is a useful information for designing appropriate clinical trials: we used low-frequency electroacupuncture in our knee osteoarthritis clinical trial; the resulting effects were significantly better than those produced by sham control and lasted for 26 weeks.2 Thus, low-frequency electroacupuncture could be useful in clinical settings to manage pain.
Summary
In summary, the studies show that electroacupuncture significantly alleviated inflammatory, neuropathic, cancer, and visceral pain in the corresponding animal models (fig. 1). Electroacupuncture intervention activates the nervous system differently in health than in pain conditions, as shown by the fact that opioid receptors are differentially involved in electroacupuncture inhibition of pain in healthy animals and those with persistent pain (table 2), as well as by the brain responses to acupuncture displayed by patients with Bell’s palsy compared with those of healthy volunteers.183 Moreover, electroacupuncture parameters affect outcomes: 10 Hz electroacupuncture produces longer-lasting alleviation of inflammatory pain than does 100 Hz, and 2 to 10 Hz inhibit nerve injury–caused allodynia/hyperalgesia more potently than does electroacupuncture at 100 Hz.
Electroacupuncture mechanisms on inflammatory pain have been extensively studied. The modality inhibits both the sensory and the affective components of inflammatory pain, acting through peripheral, spinal, and supraspinal mechanisms with the involvement of a battery of bioactive molecules including opioids, N/OFQ, serotonin, norepinephrine, glutamate receptors and transporters, cytokines, and signal molecules (table 1). Of these, opioids play a central role in electroacupuncture inhibition of all kinds of pain. Opioids desensitize peripheral nociceptors, decrease proinflammatory cytokines in peripheral sites, decrease cytokines and SP in the spinal cord, and are involved in electroacupuncture inhibition of affective pain. Opioids also activate the descending inhibitory system, the main neurotransmitters of which are serotonin and norepinephrine. Electroacupuncture induces serotonin and norepinephrine that in turn decrease GluN1 phosphorylation to inhibit pain (fig. 3).
Electroacupuncture mechanisms on neuropathic pain have been studied mainly at the spinal level, less so at the supraspinal level and peripheral sites. Most bioactive chemicals involved in electroacupuncture inhibition of inflammatory pain are also implicated in electroacupuncture inhibition of neuropathic pain (table 1 and fig. 3).
Only a few studies have investigated the mechanisms of electroacupuncture on cancer-related pain at the spinal level, but electroacupuncture has been shown to inhibit spinal SP, IL-1β, dynorphin, and transient receptor potential cation channel subfamily V member 1 to stop cancer-caused pain. Numerous articles report that electroacupuncture inhibits visceral pain through peripheral, spinal, and supraspinal mechanisms with involvement of SP, SP receptors, VIP, and VIP receptors in the colon, 5-HT, and p38 in the spinal cord, hypothalamic β-endorphin, SP, CRF, neuropeptide Y, and 5-HT in the brainstem, and NMDAR in the RVM. How these structures and bioactive molecules work together remains elusive.
Although the studies reviewed provide solid evidence for electroacupuncture analgesia, the current research has some shortcomings. First, brain mechanisms underpinning electroacupuncture analgesia are less studied than are peripheral and spinal mechanisms. Although many nuclei have been found to be involved in electroacupuncture analgesia, it is not clear how they work together in electroacupuncture inhibition of persistent pain. For example, the involvement of ACC afferents and efferents in electroacupuncture inhibition of pain has not been clarified. Given that neuronal electrical activities play important roles in the functional connection of neuronal circuits, how electroacupuncture modulates neuronal activities during persistent pain warrants investigation; imaging and electrophysiological studies may be used to explore how electroacupuncture modulates neuronal circuit connections. Second, as summarized in table 1, although many bioactive chemicals in the spinal cord participate in electroacupuncture inhibition of inflammatory and neuropathic pain, it is unclear whether these chemicals play similar roles in the brain; the peripheral mechanisms of electroacupuncture inhibition of neuropathic pain and the mechanisms of electroacupuncture inhibition of cancer pain are not well studied. Third, female rats have been seldom used in electroacupuncture analgesia studies. Because there is strong evidence for sex differences in pain and treatment outcomes, female rats should be included in future acupuncture studies to determine whether males and females respond differently to acupuncture/electroacupuncture treatment. Clarification of acupuncture/electroacupuncture mechanisms will open a variety of opportunities to combine acupuncture/electroacupuncture with medications to manage and control pain, which makes it all the more important to continue such research.

needle stimulator and acupuncture point detector




++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++
e - IC Acupuncture XO___XO
e - IC Acupuncture XO___XO


+++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++











Thanks for sharing nice information about pain relief digital acupuncture therapy device with us. i glad to read this post.
BalasHapusI have read the blog and I will follow your blogging idea.
BalasHapusThanks for your kind blog.It is so follow able.
acupuncture for back pain | shoulder pain acupuncture