


The Cyborg Era Begins

cyborg technology is a robotic solution to attach the artificial body parts to the humans.The artificial body parts attached to human body will be under the control of human brain.
and work like a natural body parts.

A cyborg is a cybernetic organism. The term cyborg is defined as an organism that is a self-regulating integration of artificial and natural systems. A human cyborg is a human who has certain physiological processes aided or controlled by mechanical or electronic devices.
- 1)convenient cyborg: external provision of an exoskeleton for the satisfying the altered fancy needs of body. Best example of convenient cyborg.
- . Best example for conditional cyborg. conditional cyborg includes bionic implants replanting the lost or damage body for the normal living in the present environment..
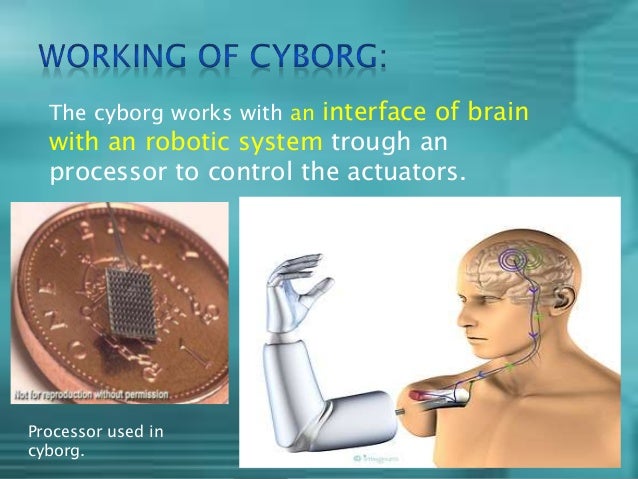
- . The cyborg works with an interface of brain with an robotic system trough an processor to control the actuators. Processor used in cyborg.
- Development of “cyborg soldier”. A soldier whose weapons as well as the survival systems are integrated into the self creating a human- machine interface. Military man with cyborg exoskeleton.
- Replacement of body organs with the artificial one like Bionic eyes, ears, hearts, lungs, kidneys, livers, hands, feets, legs, arms and skin are now real science rather than concept designs. An interesting idea is how the notion of a cyborg might change (often imagined as fusion of mechanical and electronic technology with human biology).
- . Worlds first artificial stomach Artificial muscles Artificial bone marrow skin
- . Artificial heart touch bones i-limbArtificial eye
- Prolongs life. Enables one to lead a normal life. Gives a part of the body back. Improves the quality of life. Gets a hope on life.
- Training is needed. This are very expensive. Psychological problems. Feeling different to everyone else. The risk of infections. Weekly monitoring is needed. Pain during operation.
- It seems that in future we may have more and more flexible, cheap, less weighted and less sized internal body parts may available It act as a boom to human body by improving the quality and the life. But it act as a curse.

Electronic components are invading the body. In the lab, cling wrap–like circuitry draped over the hearts of test animals can track the activity of each of the heart's four chambers and kill tissue that initiate potentially deadly arrhythmias. Other arrays penetrate brain tissue to monitor the abnormal nerve firing patterns in epilepsy or induce gene expression in the brain tissue of mice. One team has even made a 3D printed bionic ear able to pick up ultrasonic bleats that humans cannot hear. These early prototypes can't produce science-fiction cyborgs; most are used in medicine. But over time, expect devices that will make us better athletes and soldiers, or even improve our complexions.

Cyborg Intelligence: Recent Progress and Future Directions
The combination of biological and artificial intelligence is a promising methodology to construct a novel intelligent modality, proposed as cyborg intelligence. The hierarchical conceptual framework is based on the interaction and combination of comparable components of biological cognitive units and computing intelligent units. The authors extend the previous conceptual framework and focus on sensorimotor circuits to explore the representation and integration of sensation. They then present a cognitive computing model for brain-computer integration and design efficient machine-learning algorithms for neural signal decoding. They also propose biological reconstruction methods for sensorimotor circuits to not only restore but enhance functionalities with AI. They develop a series of demonstrating systems to validate the conceptual framework of cyborg intelligence and possibly herald bright prospects and high values in diversified aspects of theoretical research, engineering techniques, and clinical applications. Finally, the authors summarize the latest research trends and challenges, which they believe will further boost new scientific frontiers in cyborg intelligence .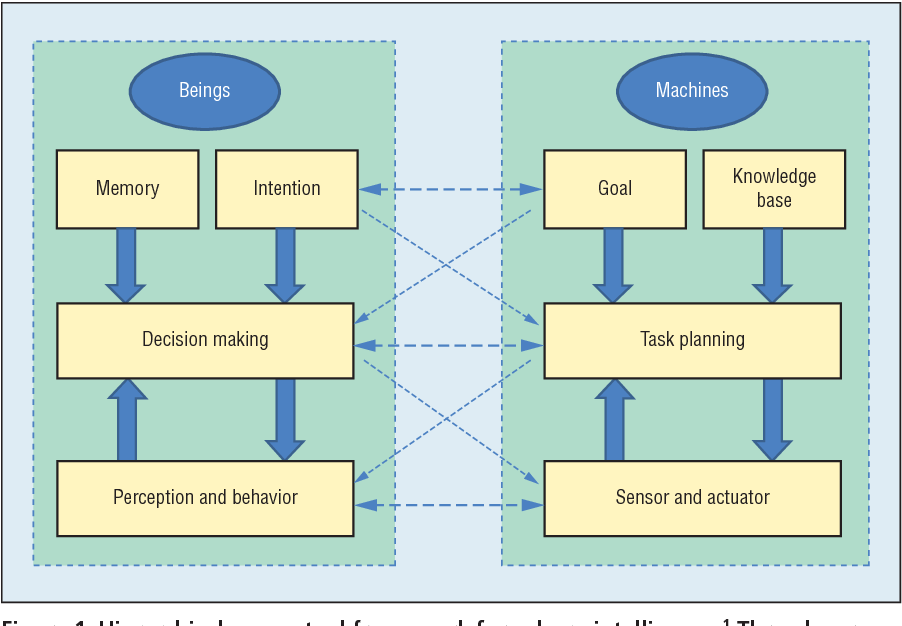
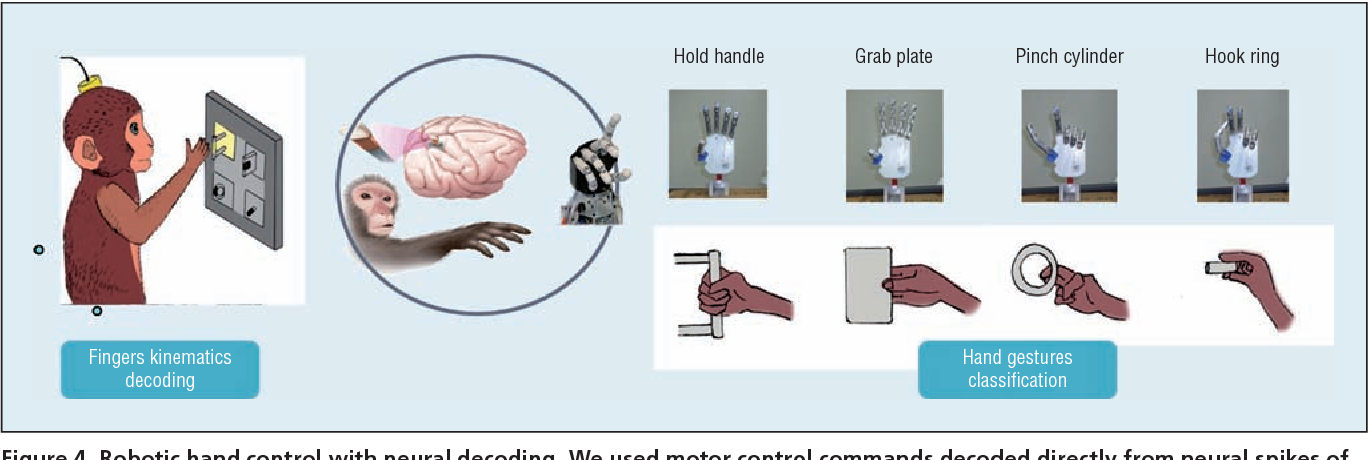
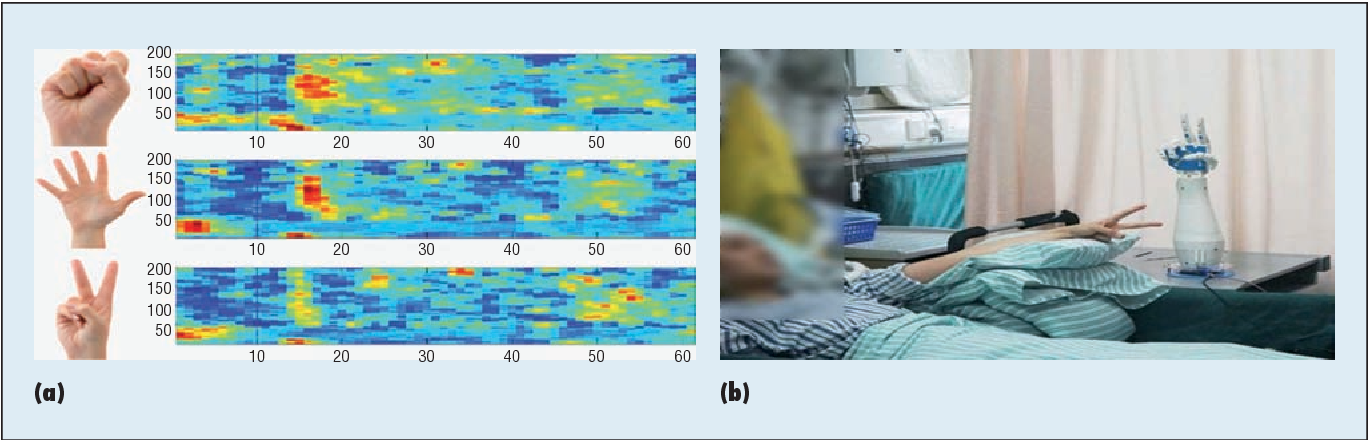





These electronics can monitor the brain, then dissolve and vanish

From pacemakers to insulin pumps, electronic implants are a powerful medical tool, but they present their own suite of risks — scarring, rejection and sepsis among them. Now a team of researchers has created a dissolving electronic implant, sort of like a much more sophisticated version of dissolving sutures. Sutures, however, can’t be injected into a rat’s brain, and don’t come equipped with temporary Wi-Fi.
The research team is calling the implantable chips “bioresorbable.” These tiny chips are biodegradable in the fluid environment of a living creature: They dissolve after a few days. The chips are made of biologically inert materials like silicon, or similar materials that won’t cause an immune response or an overdose. In rats, the researchers successfully implanted microchips that measured temperature and pressure from inside the brain. That kind of information is critical for monitoring swelling and inflammation as patients recover from a brain injury or surgery.
These dissolving implantable microchips are made out of tiny, flexible piezoresistive sensors. Under mechanical forces, the electrical resistance of the sensor body changes, which allows them to function as reliable pressure sensors. Piezoresistive sensors are also exquisitely dependent on temperature, so they make sensitive implantable thermometers. The sensor is connected to a flake of silicon sufficient to parse and transmit the information through molybdenum wires that run to a little wireless transmitter module implanted below the skin. The whole sensor chip is coated with silicon, magnesium (of which we have an RDA, or Recommended Daily Allowance of about eight of these chips per day) and a dissolvable copolymer called PLGA that we’re already using in other medical devices.
As a proof of concept, the chips stayed viable in various rat body cavities and fluids including cerebrospinal fluid for several days, while the rats ambled freely around their habitats. Longevity of the implant is, in part, a function of the thickness of the coating: the thicker the coating, the longer the chip takes to dissolve. Researchers are hoping to make versions of these bioresorbable implants that can last for much longer — perhaps the whole duration of a patient’s treatment.
Sensors of this sort have the potential to revolutionize patient treatment. While we have medical technologies that allow us to image what’s going on inside a body, our ability to directly observe the internal organs is limited by many factors. Microscopic sensors that can report shifts in temperature and pressure could map the damaged areas of a stroke victim’s brain far more accurately than any technology we have today.
Injectable electronics unfold to monitor brain activity
Electronics a million times more flexible than the best currently available could transform patient monitoring
Flexible electronics have been created that unfold once injected into a biological tissue such as the brain. The US team behind this invention were able to use the mesh electronics to map brain activity in rodents paving the way for similar tests in humans. The work could lead to a new generation of biomedical and implantable electronics that can be easily introduced without damaging surrounding tissue.
Flexible electronics have been incorporated into both living and non-living 3D structures before. But as they have traditionally been continuous sheets and relatively rigid, they required an opening equal to their size in order to be implanted, which defeats many of the advantages of flexible electronics. Furthermore, the mechanical mismatch between traditional thin film flexible electronics and neural tissue frequently produced a chronic immune response.

ultra-flexible electronics – around a million times more supple than state of the art flexible electronics – with a macroporous structure like chicken-wire and shown that it can be rolled up inside a syringe and injected into tissues and other 3D structures. Once injected, the electronic mesh, which can be up to 30 times the width of the needle, unfolds.
similar macroporous electronics made from biocompatible polymers could be used as a scaffold for tissue engineering in order to create tissue innervated with electronic sensors. They then wondered how they could deliver the electronics to existing tissue.
‘When releasing the mesh electronics from the fabrication substrate, we noticed that it was almost invisible and very flexible like a polymer and we could use a pipette to transfer from one solution to another,’ explains Lieber. ‘From there, we simply asked, would it be possible to deliver the mesh electronics by syringe-needle injection, a process common to delivery of many species in biology and medicine?’
Another fine mesh
To find an answer, the team designed and fabricated mesh-like electronics containing different sensors and electronic units on a 2D substrate by standard lithography. Since the substrate had a layer that dissolves in solution to release the mesh electronics, the electronics could subsequently be loaded into a syringe with metal or glass needles.
From there, the team injected piezoresistive electronics into polymer structures and were able to monitor mechanical strain, showing how this approach could track structural deficiencies in engineered materials. The team then injected electronics into the brains of live mice, which seamlessly integrated with the tissue while showing no immune response, and could reliably monitor brain activity.
‘Since no one had done this before, the fact that you could do such an injection – treating electronics as something like a reagent or drug – without damaging the circuitry or the targeted structures was really cool ! . Even months after the electronics were implanted in the brains of the rodents, the team saw no scar tissue or immune response. ‘This contrasts all work to date with larger and more rigid probes – it is a great result because it could be transformative for brain science and medicine.’
Self healing lithium-ion battery developed
A power source that can repair itself could be used for wearable, tearable electronics
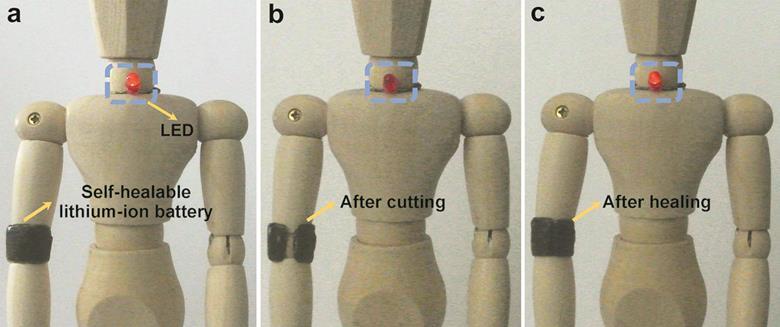
self-healing, flexible batteries that could one day find use in wearable electronic devices.
Wearable technology, such as activity trackers, augmented reality glasses and even light-up Bluetooth cocktail dresses, is growing in popularity. However, developing durable power sources that can safely withstand the strains of everyday activities remains a challenge.
flexible lithium-ion batteries that can grow back together after being broken apart, without losing their electrochemical functionality.
The batteries are formed of layers of carbon nanotubes which are loaded with lithium nanoparticles and fixed onto a self-healing polymer. A cellulose-based gel acts both as an electrolyte and separating layer between the electrodes. After being cut in two, the battery can self-repair when the two sections are simply held together for a few seconds. The battery maintains its electronic performance even after several rounds of cutting and healing.
Its unique structure also prevents lithium compounds from leaking out of the electrodes after the device has sustained damage, a significant safety hazard with current products.

Digital Brain Connecting Monitor,
Cyborg Science: Ultrathin Nanowires can Monitor and Influence What Goes on Inside Your Brain

cyborg technology is bringing us tangible progress toward real-life electronic skin, prosthetics and ultra-flexible circuits. Now taking this human-machine concept to an unprecedented level, pioneering scientists are working on the seamless marriage between electronics and brain signaling with the potential to transform our understanding of how the brain works — and how to treat its most devastating diseases.
“By focusing on the nanoelectronic connections between cells, we can do things no one has done before,” says Charles M. Lieber, Ph.D. “We’re really going into a new size regime for not only the device that records or stimulates cellular activity, but also for the whole circuit. We can make it really look and behave like smart, soft biological material, and integrate it with cells and cellular networks at the whole-tissue level. This could get around a lot of serious health problems in neurodegenerative diseases in the future.”
These disorders, such as Parkinson’s, that involve malfunctioning nerve cells can lead to difficulty with the most mundane and essential movements that most of us take for granted: walking, talking, eating and swallowing.
Scientists are working furiously to get to the bottom of neurological disorders. But they involve the body’s most complex organ — the brain — which is largely inaccessible to detailed, real-time scrutiny. This inability to see what’s happening in the body’s command center hinders the development of effective treatments for diseases that stem from it.
By using nanoelectronics, it could become possible for scientists to peer for the first time inside cells, see what’s going wrong in real time and ideally set them on a functional path again.
For the past several years, Lieber has been working to dramatically shrink cyborg science to a level that’s thousands of times smaller and more flexible than other bioelectronic research efforts. His team has made ultrathin nanowires that can monitor and influence what goes on inside cells. Using these wires, they have built ultra-flexible, 3-D mesh scaffolding with hundreds of addressable electronic units, and they have grown living tissue on it. They have also developed the tiniest electronic probe ever that can record even the fastest signaling between cells.
Rapid-fire cell signaling controls all of the body’s movements, including breathing and swallowing, which are affected in some neurodegenerative diseases. And it’s at this level where the promise of Lieber’s most recent work enters the picture.
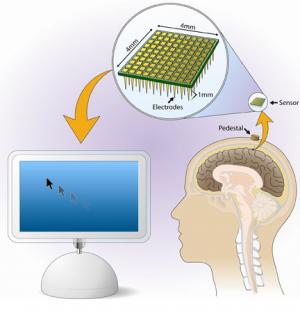
US brain-computer interface gets live human trials
Telepathic mouse kit could jump wire paralysed limbs

new "neural interface" brain-plug equipment, intended to let users control computers and machinery merely by thinking, is about to embark on its second stage of clinical trials. The tech is intended initially to help patients suffering from paralysing illness or massive injuries, but has implications beyond the medical field.

Ah, at last the caption I thought of is there.
The kit in question is called BrainGate, and is under development by neuroscience, engineering and computing boffins at Brown University in Rhode Island. It uses a grid of electrodes implanted beneath a user's skull into his or her motor cortex, linked to a "pedestal" assembly atop the cranium. At the moment, this is hooked up by a wired connection to the BrainGate computers which interpret the electrodes' readings from the motor cortex and "turn thought into action" - for instance by moving a cursor on a screen, or operating a cybernetic limb, or directing a motorised wheelchair.
Initial trials have already been carried out, according to the researchers, during which a lot was learned. However the tech is far from fully developed yet, and the second phase of trials - now beginning - is under an "investigational device exemption", meaning that the kit is still at the prototype stage and isn't certified as medically safe. Volunteers using it are acting as guinea pigs, or test pilots, as it were.
The BrainGate team has lofty goals. They note that wired connections are clumsy, and furthermore that the through-the-skin plug assembly presents a risk of infection - a rather serious one, as it penetrates not just the scalp but the skull and the protective meninges covering the brain.
Thus the team aim ultimately to design a fully internal, self-powered wireless brain implant to replace the present skull jack.
Even more impressively, they note that in many cases of paralysis - as opposed to amputation - a patient's limbs are still fully functional. They have simply been "disconnected" from the brain's motor centres by illness or damage to the nerves or spinal cord. The Brown researchers hope to find a way of using their cortical implant to send signals to other implants on undamaged nerves controlling paralysed limbs, effectively attaching jumper leads to bridge the broken nerve connections and restoring the brain's control over the relevant muscles.
In cases where the original limb is gone - the Brown scientists will be working with a lot of injured troops in this trial, as Rhode Island is home to a large US Veterans Administration research hospital - the BrainGate tech could furnish a much better and more instinctive means of controlling prosthetic limbs, powered wheelchairs etc.
Initially the tech will be strictly for medical uses, of course. But if it ever becomes truly safe and convenient to use, it isn't hard to imagine people choosing voluntarily to have a BrainGate interface implanted into their heads simply for convenience. It would confer abilities roughly equivalent to telepathy and clairvoyance right off, particularly if combined with some kind of feedback mechanism - video specs, perhaps, or an equivalent of BrainGate attached to the optic nerve instead. In a sufficiently wired and automated world, it would also be effectively telekinetic: unlocking doors, turning lights or machines on and off, handling computers etc.
Ultimately, people might choose to dispense with their flimsy, unreliable fleshy bodies and move instead to a more fulfilling and powerful life as chipped-up brains in bubbling jars - either moving around aboard robotic vehicles, or leading a vicarious existence via the future interwebs.
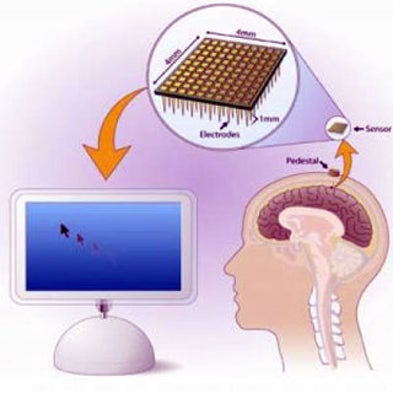
Plug and Play: Researchers Expand Clinical Study of Neural Interface Brain Implant
Having proved in 2004 that plugging a sensor into the human brain's motor cortex could turn the thoughts of paralysis victims into action, a team of Brown University scientists now has the green light from the U.S. Food and Drug Administration (FDA) and the Massachusetts General Hospital (MGH) institutional review board to expand its efforts developing technology that reconnects the brain to lifeless limbs.
Brown's BrainGate Neural Interface System—conceived in 2000 with the help of a $4.25-million U.S. Defense Department grant—includes a baby aspirin–size brain sensor containing 100 electrodes, each thinner than a human hair, that connects to the surface of the motor cortex (the part of the brain that enables voluntary movement), registers electrical signals from nearby neurons, and transmits them through gold wires to a set of computers, processors and monitors.
The researchers designed BrainGate to assist those suffering from spinal cord injuries, muscular dystrophy, brain stem stroke, amyotrophic lateral sclerosis (ALS, or Lou Gehrig's Disease), and other motor neuron diseases. During the initial testing five years ago, patients suffering from paralysis demonstrated their ability to use brain signals sent from their motor cortex to control external devices such as computer screen cursors and robotic arms just by thinking about them. "The signals may have been disconnected from the (participant's) limb, but they were still there," says Leigh Hochberg, a Brown associate professor of engineering and a vascular and critical care neurologist at MGH who is helping lead the research.
Due to the high risk of plugging a device directly into the brain, the FDA in 2004 granted the BrainGate system an investigational device exemption so that researchers could begin testing the unit in patients and collect data about its safety and effectiveness. Thanks to the success of those early tests, the researchers last week kicked off a pilot clinical trial, dubbed BrainGate2. Although the technology is similar to what was used in the original testing, the researchers are looking to enlist up to 15 patients this time and gather more information that will help them better understand brain signals as well as "the method by which we decode them," Hochberg says. Since the initial four-person clinical trial launched five years ago, "we have a better appreciation for things that we need to learn."
A successful BrainGate2 trial could open up a number of new possibilities, including the use of a second sensor to stimulate both sides of the motor cortex, says John Donoghue, a Brown neuroscience professor and director of the Brown Institute for Brain Science. Researchers thus far have implanted the sensor in the side of the brain that controls a patient's dominant side—the left cortex for righties and the right cortex for lefties.
BrainGate2 is part of a larger mission to help paralysis victims regain control of their bodies. "We want to reconnect the brain back to the muscles and eventually back to the entire limb," Donoghue says. "We are attempting to recreate parts of the nervous system that have been disconnected from the brain."
Brown's BrainGate Neural Interface System—conceived in 2000 with the help of a $4.25-million U.S. Defense Department grant—includes a baby aspirin–size brain sensor containing 100 electrodes, each thinner than a human hair, that connects to the surface of the motor cortex (the part of the brain that enables voluntary movement), registers electrical signals from nearby neurons, and transmits them through gold wires to a set of computers, processors and monitors.
The researchers designed BrainGate to assist those suffering from spinal cord injuries, muscular dystrophy, brain stem stroke, amyotrophic lateral sclerosis (ALS, or Lou Gehrig's Disease), and other motor neuron diseases. During the initial testing five years ago, patients suffering from paralysis demonstrated their ability to use brain signals sent from their motor cortex to control external devices such as computer screen cursors and robotic arms just by thinking about them. "The signals may have been disconnected from the (participant's) limb, but they were still there," says Leigh Hochberg, a Brown associate professor of engineering and a vascular and critical care neurologist at MGH who is helping lead the research.
Due to the high risk of plugging a device directly into the brain, the FDA in 2004 granted the BrainGate system an investigational device exemption so that researchers could begin testing the unit in patients and collect data about its safety and effectiveness. Thanks to the success of those early tests, the researchers last week kicked off a pilot clinical trial, dubbed BrainGate2. Although the technology is similar to what was used in the original testing, the researchers are looking to enlist up to 15 patients this time and gather more information that will help them better understand brain signals as well as "the method by which we decode them," Hochberg says. Since the initial four-person clinical trial launched five years ago, "we have a better appreciation for things that we need to learn."
A successful BrainGate2 trial could open up a number of new possibilities, including the use of a second sensor to stimulate both sides of the motor cortex, says John Donoghue, a Brown neuroscience professor and director of the Brown Institute for Brain Science. Researchers thus far have implanted the sensor in the side of the brain that controls a patient's dominant side—the left cortex for righties and the right cortex for lefties.
BrainGate2 is part of a larger mission to help paralysis victims regain control of their bodies. "We want to reconnect the brain back to the muscles and eventually back to the entire limb," Donoghue says. "We are attempting to recreate parts of the nervous system that have been disconnected from the brain."
XO___XO Cloning
Cloning, the process of generating a genetically identical copy of a cell or an organism. Cloning happens all the time in nature—for example, when a cell replicates itself asexually without any genetic alteration or recombination. Prokaryotic organisms (organisms lacking a cell nucleus) such as bacteria create genetically identical duplicates of themselves using binary fission or budding. In eukaryotic organisms (organisms possessing a cell nucleus) such as humans, all the cells that undergo mitosis, such as skin cells and cells lining the gastrointestinal tract, are clones; the only exceptions are gametes (eggs and sperm), which undergo meiosis and genetic recombination.
Early cloning experiments
Reproductive cloning was originally carried out by artificial “twinning,” or embryo splitting, which was first performed on a salamander embryo in the early 1900s by German embryologist Hans Spemann. Later, Spemann, who was awarded the Nobel Prize for Physiology or Medicine (1935) for his research on embryonic development, theorized about another cloning procedure known as nuclear transfer. This procedure was performed in 1952 by American scientists Robert W. Briggs and Thomas J. King, who used DNA from embryonic cells of the frog Rana pipiens to generate cloned tadpoles. In 1958 British biologist John Bertrand Gurdon successfully carried out nuclear transfer using DNA from adult intestinal cells of African clawed frogs (Xenopus laevis). Gurdon was awarded a share of the 2012 Nobel Prize in Physiology or Medicine for this breakthrough.
Advancements in the field of molecular biology led to the development of techniques that allowed scientists to manipulate cells and to detect chemical markers that signal changes within cells. With the advent of recombinant DNA technology in the 1970s, it became possible for scientists to create transgenic clones—clones with genomes containing pieces of DNA from other organisms. Beginning in the 1980s mammals such as sheep were cloned from early and partially differentiated embryonic cells. In 1996 British developmental biologist Ian Wilmut generated a cloned sheep, named Dolly, by means of nuclear transfer involving an enucleated embryo and a differentiated cell nucleus. This technique, which was later refined and became known as somatic cell nuclear transfer (SCNT), represented an extraordinary advance in the science of cloning, because it resulted in the creation of a genetically identical clone of an already grown sheep. It also indicated that it was possible for the DNA in differentiated somatic (body) cells to revert to an undifferentiated embryonic stage, thereby reestablishing pluripotency—the potential of an embryonic cell to grow into any one of the numerous different types of mature body cells that make up a complete organism. The realization that the DNA of somatic cells could be reprogrammed to a pluripotent state significantly impacted research into therapeutic cloning and the development of stem cell therapies.
Soon after the generation of Dolly, a number of other animals were cloned by SCNT, including pigs, goats, rats, mice, dogs, horses, and mules. Despite those successes, the birth of a viable SCNT primate clone would not come to fruition until 2018, and scientists used other cloning processes in the meantime. In 2001 a team of scientists cloned a rhesus monkey through a process called embryonic cell nuclear transfer, which is similar to SCNT except that it uses DNA from an undifferentiated embryo. In 2007 macaque monkey embryos were cloned by SCNT, but those clones lived only to the blastocyst stage of embryonic development. It was more than 10 years later, after improvements to SCNT had been made, that scientists announced the live birth of two clones of the crab-eating macaque (Macaca fascicularis), the first primate clones using the SCNT process. (SCNT has been carried out with very limited success in humans, in part because of problems with human egg cells resulting from the mother’s age and environmental factors.)

Reproductive cloning
Reproductive cloning involves the implantation of a cloned embryo into a real or an artificial uterus. The embryo develops into a fetus that is then carried to term. Reproductive cloning experiments were performed for more than 40 years through the process of embryo splitting, in which a single early-stage two-cell embryo is manually divided into two individual cells and then grows as two identical embryos. Reproductive cloning techniques underwent significant change in the 1990s, following the birth of Dolly, who was generated through the process of SCNT. This process entails the removal of the entire nucleus from a somatic (body) cell of an organism, followed by insertion of the nucleus into an egg cell that has had its own nucleus removed (enucleation). Once the somatic nucleus is inside the egg, the egg is stimulated with a mild electrical current and begins dividing. Thus, a cloned embryo, essentially an embryo of an identical twin of the original organism, is created. The SCNT process has undergone significant refinement since the 1990s, and procedures have been developed to prevent damage to eggs during nuclear extraction and somatic cell nuclear insertion. For example, the use of polarized light to visualize an egg cell’s nucleus facilitates the extraction of the nucleus from the egg, resulting in a healthy, viable egg and thereby increasing the success rate of SCNT.

Reproductive cloning using SCNT is considered very harmful since the fetuses of embryos cloned through SCNT rarely survive gestation and usually are born with birth defects. Wilmut’s team of scientists needed 277 tries to create Dolly. Likewise, attempts to produce a macaque monkey clone in 2007 involved 100 cloned embryos, implanted into 50 female macaque monkeys, none of which gave rise to a viable pregnancy. In January 2008, scientists at Stemagen, a stem cell research and development company in California, announced that they had cloned five human embryos by means of SCNT and that the embryos had matured to the stage at which they could have been implanted in a womb. However, the scientists destroyed the embryos after five days, in the interest of performing molecular analyses on them.
Therapeutic cloning
Therapeutic cloning is intended to use cloned embryos for the purpose of extracting stem cells from them, without ever implanting the embryos in a womb. Therapeutic cloning enables the cultivation of stem cells that are genetically identical to a patient. The stem cells could be stimulated to differentiate into any of the more than 200 cell types in the human body. The differentiated cells then could be transplanted into the patient to replace diseased or damaged cells without the risk of rejection by the immune system. These cells could be used to treat a variety of conditions, including Alzheimer disease, Parkinson disease, diabetes mellitus, stroke, and spinal cord injury. In addition, stem cells could be used for in vitro (laboratory) studies of normal and abnormal embryo development or for testing drugs to see if they are toxic or cause birth defects.

Foundations of Molecular Cloning - Past, Present and Future
Introduction
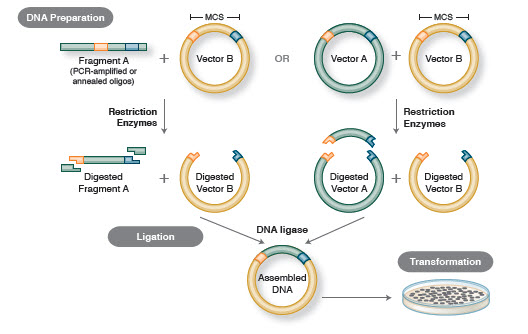
Molecular cloning refers to the isolation of a DNA sequence from any species (often a gene), and its insertion into a vector for propagation, without alteration of the original DNA sequence. Once isolated, molecular clones can be used to generate many copies of the DNA for analysis of the gene sequence, and/or to express the resulting protein for the study or utilization of the protein’s function. The clones can also be manipulated and mutated in vitro to alter the expression and function of the protein.The basic cloning workflow includes four steps:
- Isolation of target DNA fragments (often referred to as inserts)
- Ligation of inserts into an appropriate cloning vector, creating recombinant molecules (e.g., plasmids)
- Transformation of recombinant plasmids into bacteria or other suitable host for propagation
- Screening/selection of hosts containing the intended recombinant plasmid
History of Cloning
The Foundation of Molecular Cloning
Cutting (Digestion). Recombinant DNA technology first emerged in the late 1960s, with the discovery of enzymes that could specifically cut and join double-stranded DNA molecules. In fact, as early as 1952, two groups independently observed that bacteria encoded a “restriction factor” that prevented bacteriophages from growing within certain hosts (1,2). But the nature of the factor was not discovered until 1968, when Arber and Linn succeeded in isolating an enzyme, termed a restriction factor, that selectively cut exogenous DNA, but not bacterial DNA (3). These studies also identified a methylase enzyme that protected the bacterial DNA from restriction enzymes.Shortly after Arber and Linn’s discovery, Smith extended and confirmed these studies by isolating a restriction enzyme from Haemophilus influenza. He demonstrated that the enzyme selectively cut DNA in the middle of a specific 6 base-pair stretch of DNA; one characteristic of certain restriction enzymes is their propensity to cut the DNA substrate in or near specific, often palindromic, “recognition” sequences (4).
The full power of restriction enzymes was not realized until restriction enzymes and gel electrophoresis were used to map the Simian Virus 40 (SV40) genome (5). For these seminal findings, Werner Arber, Hamilton Smith, and Daniel Nathans shared the 1978 Nobel Prize in Medicine.
Figure 1. Traditional Cloning Workflow
Not long after the discovery of restriction enzymes and DNA ligases, the first recombinant DNA molecule was made. In 1972, Berg separately cut and ligated a piece of lambda bacteriophage DNA or the E. coli galactose operon with SV40 DNA to create the first recombinant DNA molecules (14). These studies pioneered the concept that, because of the universal nature of DNA, DNA from any species could be joined together. In 1980, Paul Berg shared the Nobel Prize in Chemistry with Walter Gilbert and Frederick Sanger (the developers of DNA sequencing), for “his fundamental studies of the biochemistry of nucleic acids, with particular regard to recombinant DNA.”
Transformation. Recombinant DNA technology would be severely limited, and molecular cloning impossible, without the means to propagate and isolate the newly constructed DNA molecule. The ability to transform bacteria, or induce the uptake, incorporation and expression of foreign genetic material, was first demonstrated by Griffith when he transformed a non-lethal strain of bacteria into a lethal strain by mixing the non-lethal strain with heat-inactivated lethal bacteria (15). However, the nature of the “transforming principle” that conveyed lethality was not understood until 1944. In the same year, Avery, Macleod and McCarty demonstrated that DNA, and not protein, was responsible for inducing the lethal phenotype (16).
Initially, it was believed that the common bacterial laboratory strain, E. coli, was refractory to transformation, until Mandel and Higa demonstrated that treatment of E. coli with calcium chloride induced the uptake of bacteriophage DNA (17). Cohen applied this principle, in 1972, when he pioneered the transformation of bacteria with plasmids to confer antibiotic resistance on the bacteria (18).
The ultimate experiment: digestion, ligation and transformation of a recombinant DNA molecule was executed by Boyer, Cohen and Chang in 1973, when they digested the plasmid pSC101 with EcoRI, ligated the linearized fragment to another enzyme-restricted plasmid and transformed the resulting recombinant molecule into E. coli, conferring tetracycline resistance on the bacteria (19), thus laying the foundation for most recombinant DNA work since.
Building on the Groundwork
While scientists had discovered and applied all of the basic principles for creating and propagating recombinant DNA in bacteria, the process was inefficient. Restriction enzyme preparations were unreliable due to non-standardized purification procedures, plasmids for cloning were cumbersome, difficult to work with and limited in number, and experiments were limited by the amount of insert DNA that could be isolated. Research over the next few decades led to improvements in the techniques and tools available for molecular cloning.Early vector design.
Development of the first standardized vector. Scientists working in Boyer’s lab recognized the need for a general cloning plasmid, a compact plasmid with unique restriction sites for cloning in foreign DNA and the expression of antibiotic resistance genes for selection of transformed bacteria. In 1977, they described the first vector designed for cloning purposes, pBR322 (20). This vector was small, ~4 kilobases in size, and had two antibiotic resistance genes for selection.
Vectors with on-board screening and higher yields. Although antibiotic selection prevented non-transformed bacteria from growing, plasmids that re-ligated without insert DNA fragments (self-ligation) could still confer antibiotic resistance on bacteria. Therefore, finding the correct bacterial clones containing the desired recombinant DNA molecule could be time-consuming.
Vieira and Messing devised a screening tool to identify bacterial colonies containing plasmids with DNA inserts. Based upon the pBR322 plasmid, they created the series of pUC plasmids, which contained a “blue/white screening” system (21). Placement of a multiple cloning site (MCS) containing several unique restriction sites within the LacZ´ gene allowed researchers to screen for bacterial colonies containing plasmids with the foreign DNA insert. When bacteria were plated on the correct media, white colonies contained plasmids with inserts, while blue colonies contained plasmids with no inserts. pUC plasmids had an additional advantage over existing vectors; they contained a mutation that resulted in higher copy numbers, therefore increasing plasmid yields.
Improving restriction digests. Early work with restriction enzymes was hampered by the purity of the enzyme preparation and a lack of understanding of the buffer requirements for each enzyme. In 1975, New England Biolabs (NEB) became the first company to commercialize restriction enzymes produced from a recombinant source. This enabled higher yields, improved purity, lot-to-lot consistency and lower pricing. Currently, over 4,000 restriction enzymes, recognizing over 300 different sequences, have been discovered by scientists across the globe [for a complete list of restriction enzymes and recognition sequences, visit REBASE® at rebase.neb.com (22)]. NEB currently supplies over 230 of these specificities.
NEB was also one of the first companies to develop a standardized four-buffer system, and to characterize all of its enzyme activities in this buffer system. This led to a better understanding of how to conduct a double digest, or the digestion of DNA with two enzymes sim-ultaneously. Later research led to the development of one-buffer systems, which are compatible with the most common restriction enzymes (such as NEB’s CutSmart™ Buffer).
With the advent of commercially available restriction enzyme libraries with known sequence specificities, restriction enzymes became a powerful tool for screening potential recombinant DNA clones. The “diagnostic digest” was, and still is, one of the most common techniques used in molecular cloning.
Vector and insert preparation. Cloning efficiency and versatility were also improved by the development of different techniques for preparing vectors prior to ligation. Alkaline phosphatases were isolated that could remove the 3´ and 5´ phosphate groups from the ends of DNA [and RNA; (23)]. It was soon discovered that treatment of vectors with Calf-Intestinal Phosphatase (CIP) dephosphorylated DNA ends and prevented self-ligation of the vector, increasing recovery of plasmids with insert (24).
The CIP enzyme proved difficult to inactivate, and any residual activity led to dephosphorylation of insert DNA and inhibition of the ligation reaction. The discovery of the heat-labile alkaline phosphatases, such as recombinant Shrimp Alkaline Phosphatase (rSAP) and Antarctic Phosphatase (AP) (both sold by NEB), decreased the steps and time involved, as a simple shift in temperature inactivates the enzyme prior to the ligation step (25).
DNA sequencing arrives. DNA sequencing was developed in the late 1970s when two competing methods were devised. Maxam and Gilbert developed the “chemical sequencing method,” which relied on chemical modification of DNA and subsequent cleavage at specific bases (26). At the same time, Sanger and colleagues published on the “chain-termination method”, which became the method used by most researchers (27). The Sanger method quickly became automated, and the first automatic sequencers were sold in 1987.
The ability to determine the sequence of a stretch of DNA enhanced the reliability and versatility of molecular cloning. Once cloned, scientists could sequence clones to definitively identify the correct recombinant molecule, identify new genes or mutations in genes, and easily design oligonucleotides based on the known sequence for additional experiments.
The impact of the polymerase chain reaction. One of the problems in molecular cloning in the early years was obtaining enough insert DNA to clone into the vector. In 1983, Mullis devised a technique that solved this problem and revolutionized molecular cloning (28). He amplified a stretch of target DNA by using opposing primers to amplify both complementary strands of DNA, simultaneously. Through cycles of denaturation, annealing and polymerization, he showed he could exponentially amplify a single copy of DNA. The polymerase chain reaction, or PCR, made it possible to amplify and clone genes from previously inadequate quantities of DNA. For this discovery, Kary Mullis shared the 1993 Nobel Prize in Chemistry “for contributions to the developments of methods within DNA-based chemistry”.
In 1970, Temin and Baltimore independently discovered reverse transcriptase in viruses, an enzyme that converts RNA into DNA (29,30). Shortly after PCR was developed, reverse transcription was coupled with PCR (RT-PCR) to allow cloning of messenger RNA (mRNA). Reverse transcription was used to create a DNA copy (cDNA) of mRNA that was subsequently amplified by PCR to create an insert for ligation. For their discovery of the enzyme, Howard Temin and David Baltimore were awarded the 1975 Nobel Prize in Medicine and Physiology, which they shared with Renato Dulbecco.
Cloning of PCR products. The advent of PCR meant that researchers could now clone genes and DNA segments with limited knowledge of amplicon sequence. However, there was little consensus as to the optimal method of PCR product preparation for efficient ligation into cloning vectors.
Several different methods were initially used for cloning PCR products. The simplest, and still the most common, method for cloning PCR products is through the introduction of restriction sites onto the ends of the PCR product (31). This allows for direct, directional cloning of the insert into the vector after restriction digestion. Blunt-ended cloning was developed to directly ligate PCR products generated by polymerases that produced blunt ends, or inserts engineered to have restriction sites that left blunt ends once the insert was digested. This was useful in cloning DNA fragments that did not contain restriction sites compatible with the vector (32).
Shortly after the introduction of PCR, overlap extension PCR was introduced as a method to assemble PCR products into one contiguous DNA sequence (33). In this method, the DNA insert is amplified by PCR using primers that generate a PCR product containing overlapping regions with the vector. The vector and insert are then mixed, denatured and annealed, allowing hybridization of the insert to the vector. A second round of PCR generates recombinant DNA molecules of insert-containing vector. Overlap extension PCR enabled researchers to piece together large genes that could not easily be amplified by traditional PCR methods. Overlap extension PCR was also used to introduce mutations into gene sequences (34).
Figure 2. Overview of PCR

In an effort to further improve the efficiency of molecular cloning, several specialized tools and techniques were developed that exploited the properties of unique enzymes.
TA Cloning. One approach took advantage of a property of Taq DNA Polymerase, the first heat-stable polymerase used for PCR. During amplification, Taq adds a single 3´ dA nucleotide to the end of each PCR product. The PCR product can be easily ligated into a vector that has been cut and engineered to contain single T residues on each strand. Several companies have marketed the technique and sell kits containing cloning vectors that are already linearized and “tailed”.
LIC. Ligation independent cloning (LIC), as its name implies, allows for the joining of DNA molecules in the absence of DNA ligase. LIC is commonly performed with T4 DNA Polymerase, which is used to generate single-stranded DNA overhangs, >12 nucleotides long, onto both the linearized vector DNA and the insert to be cloned (35). When mixed together, the vector and insert anneal through the long stretch of compatible ends. The length of the compatible ends is sufficient to hold the molecule together in the absence of ligase, even during transformation. Once transformed, the gaps are repaired in vivo. There are several different commercially available products for LIC. USER cloning.
USER cloning was first developed in the early 1990s as a restriction enzyme- and ligase-independent cloning method (36). When first conceived, the method relied on using PCR primers that contained a ~12 nucleotide 5´ tail, in which at least four deoxythymidine bases had been substituted with deoxyuridines. The PCR product was treated with uracil DNA glycosidase (UDG) and Endonuclease VIII, which excises the uracil bases and leaves a 3´ overlap that can be annealed to a similarly treated vector. NEB sells the USER enzyme for ligase and restriction enzyme independent cloning reactions.
Future Trends
Molecular cloning has progressed from the cloning of a single DNA fragment to the assembly of multiple DNA components into a single contiguous stretch of DNA. New and emerging technologies seek to transform cloning into a process that is as simple as arranging “blocks” of DNA next to each other.DNA assembly methods. Many new, elegant technologies allow for the assembly of multiple DNA fragments in a one-tube reaction. The advantages of these technologies are that they are standardized, seamless and mostly sequence independent. In addition, the ability to assemble multiple DNA fragments in one tube turns a series of previously independent restriction/ligation reactions into a streamlined, efficient procedure.
Different techniques and products for gene assembly include SLIC (Sequence and Ligase Independent Cloning), Gibson Assembly (NEB), GeneArt® Seamless Cloning (Life Technologies) and Gateway® Cloning (Invitrogen) (35,37,38).
In DNA assembly, blocks of DNA to be assembled are PCR amplified. Then, the DNA fragments to be assembled adjacent to one another are engineered to contain blocks of complementary sequences that will be ligated together. These could be compatible cohesive ends, such as those used for Gibson Assembly, or regions containing recognition sites for site-specific recombinases (Gateway). The enzyme used for DNA ligation will recognize and assemble each set of compatible regions, creating a single, contiguous DNA molecule in one reaction.
Figure 3. Overview of the Gibson Assembly Cloning Method
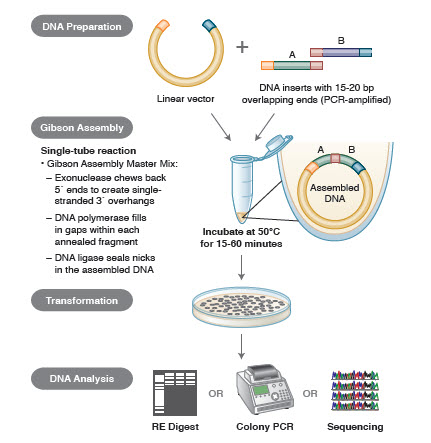
Conclusion
In the last 40 years, molecular cloning has progressed from arduously isolating and piecing together two pieces of DNA, followed by intensive screening of potential clones, to seamlessly assembling up to 10 DNA fragments with remarkable efficiency in just a few hours, or designing DNA molecules in silico and synthesizing them in vitro. Together, all of these technologies give molecular biologists an astonishingly powerful toolbox for exploring, manipulating and harnessing DNA, that will further broaden the horizons of science. Among the possibilities are the development of safer recombinant proteins for the treatment of diseases, enhancement of gene therapy (43), and quicker production, validation and release of new vaccines (44). But ultimately, the potential is constrained only by our imaginations.Surface plasmon resonance
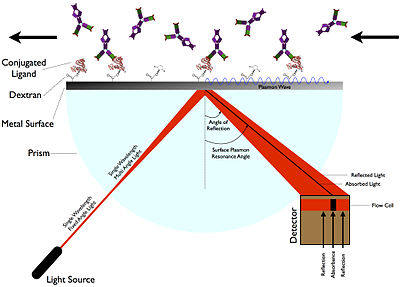
Surface plasmon resonance (SPR) is the resonant oscillation of conduction electrons at the interface between negative and positive permittivity material stimulated by incident light. SPR is the basis of many standard tools for measuring adsorption of material onto planar metal (typically gold or silver) surfaces or onto the surface of metal nanoparticles. It is the fundamental principle behind many color-based biosensor applications, different lab-on-a-chip sensors and diatom photosynthesis.
Explanation
The surface plasmon polariton is a non-radiative electromagnetic surface wave that propagates in a direction parallel to the negative permittivity/dielectric material interface. Since the wave is on the boundary of the conductor and the external medium (air, water or vacuum for example), these oscillations are very sensitive to any change of this boundary, such as the adsorption of molecules to the conducting surface.To describe the existence and properties of surface plasmon polaritons, one can choose from various models (quantum theory, Drude model, etc.). The simplest way to approach the problem is to treat each material as a homogeneous continuum, described by a frequency-dependent relative permittivity between the external medium and the surface. This quantity, hereafter referred to as the materials' "dielectric function", is the complex permittivity. In order for the terms that describe the electronic surface plasmon to exist, the real part of the dielectric constant of the conductor must be negative and its magnitude must be greater than that of the dielectric. This condition is met in the infrared-visible wavelength region for air/metal and water/metal interfaces (where the real dielectric constant of a metal is negative and that of air or water is positive).
LSPRs (Localized SPRs) are collective electron charge oscillations in metallic nanoparticles that are excited by light. They exhibit enhanced near-field amplitude at the resonance wavelength. This field is highly localized at the nanoparticle and decays rapidly away from the nanoparticle/dieletric interface into the dielectric background, though far-field scattering by the particle is also enhanced by the resonance. Light intensity enhancement is a very important aspect of LSPRs and localization means the LSPR has very high spatial resolution (subwavelength), limited only by the size of nanoparticles. Because of the enhanced field amplitude, effects that depend on the amplitude such as magneto-optical effect are also enhanced by LSPRs.
Implementations
Otto configuration
Kretschmann configuration
 is the relative permittivity, and
is the relative permittivity, and  is the relative permeability of the material (1: the glass block, 2: the metal film), while
is the relative permeability of the material (1: the glass block, 2: the metal film), while  is angular frequency and
is angular frequency and  is the speed of light in a vacuum.
is the speed of light in a vacuum.
Typical metals that support surface plasmons are silver and gold, but metals such as copper, titanium or chromium have also been used.
When using light to excite SP waves, there are two configurations which are well known. In the Otto setup, the light illuminates the wall of a glass block, typically a prism, and is totally internally reflected. A thin metal film (for example gold) is positioned close enough to the prism wall so that an evanescent wave can interact with the plasma waves on the surface and hence excite the plasmons.
In the Kretschmann configuration, the metal film is evaporated onto the glass block. The light again illuminates the glass block, and an evanescent wave penetrates through the metal film. The plasmons are excited at the outer side of the film. This configuration is used in most practical applications.
SPR emission
When the surface plasmon wave interacts with a local particle or irregularity, such as a rough surface, part of the energy can be re-emitted as light. This emitted light can be detected behind the metal film from various directions.Applications
Surface plasmons have been used to enhance the surface sensitivity of several spectroscopic measurements including fluorescence, Raman scattering, and second harmonic generation. However, in their simplest form, SPR reflectivity measurements can be used to detect molecular adsorption, such as polymers, DNA or proteins, etc. Technically, it is common to measure the angle of minimum reflection (angle of maximum absorption). This angle changes in the order of 0.1° during thin (about nm thickness) film adsorption. (See also the Examples.) In other cases the changes in the absorption wavelength is followed. The mechanism of detection is based on that the adsorbing molecules cause changes in the local index of refraction, changing the resonance conditions of the surface plasmon waves. The same principle is exploited in the recently developed competitive platform based on loss-less dielectric multilayers (DBR), supporting surface electromagnetic waves with sharper resonances (Bloch surface waves).
If the surface is patterned with different biopolymers, using adequate optics and imaging sensors (i.e. a camera), the technique can be extended to surface plasmon resonance imaging (SPRI). This method provides a high contrast of the images based on the adsorbed amount of molecules, somewhat similar to Brewster angle microscopy (this latter is most commonly used together with a Langmuir–Blodgett trough).
For nanoparticles, localized surface plasmon oscillations can give rise to the intense colors of suspensions or sols containing the nanoparticles. Nanoparticles or nanowires of noble metals exhibit strong absorption bands in the ultraviolet-visible light regime that are not present in the bulk metal. This extraordinary absorption increase has been exploited to increase light absorption in photovoltaic cells by depositing metal nanoparticles on the cell surface.The energy (color) of this absorption differs when the light is polarized along or perpendicular to the nanowire. Shifts in this resonance due to changes in the local index of refraction upon adsorption to the nanoparticles can also be used to detect biopolymers such as DNA or proteins. Related complementary techniques include plasmon waveguide resonance, QCM, extraordinary optical transmission, and dual polarization interferometry.
SPR Immunoassay
The first SPR immunoassay was proposed in 1983 by Liedberg, Nylander, and Lundström, then of the Linköping Institute of Technology (Sweden). They adsorbed human IgG onto a 600-angstrom silver film, and used the assay to detect anti-human IgG in water solution. Unlike many other immunoassays, such as ELISA, an SPR immunoassay is label free in that a label molecule is not required for detection of the analyte. Additionally, the measurements on SPR can be followed real-time allowing the monitoring of individual steps in sequential binding events particularly useful in the assessment of for instance sandwich complexes.Material characterization
Multi-Parametric Surface Plasmon Resonance, a special configuration of SPR, can be used to characterize layers and stacks of layers. Besides binding kinetics, MP-SPR can also provide information on structural changes in terms of layer true thickness and refractive index. MP-SPR has been applied successfully in measurements of lipid targeting and rupture, CVD-deposited single monolayer of graphene (3.7Å) as well as micrometer thick polymers.Data interpretation
The most common data interpretation is based on the Fresnel formulas, which treat the formed thin films as infinite, continuous dielectric layers. This interpretation may result in multiple possible refractive index and thickness values. However, usually only one solution is within the reasonable data range. In Multi-Parametric Surface Plasmon Resonance, two SPR curves are acquired by scanning a range of angles at two different wavelengths, which results in a unique solution for both thickness and refractive index.Metal particle plasmons are usually modeled using the Mie scattering theory.
In many cases no detailed models are applied, but the sensors are calibrated for the specific application, and used with interpolation within the calibration curve.
Examples
Layer-by-layer self-assembly
SPR curves measured during the adsorption of a polyelectrolyte and then a clay mineral self-assembled film onto a thin (ca. 38 nanometers) gold sensor.
When higher speed observation is desired, one can select an angle right below the resonance point (the angle of minimum reflectance), and measure the reflectivity changes at that point. This is the so-called 'dynamic SPR' measurement. The interpretation of the data assumes that the structure of the film does not change significantly during the measurement.
Binding constant determination
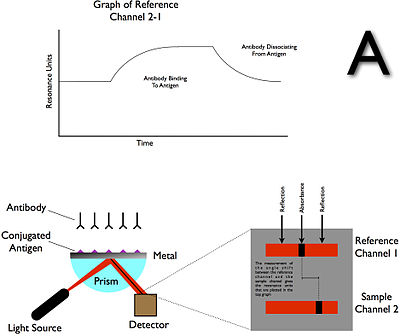
Association and dissociation signal
Example of output from Biacore
For this, a bait ligand is immobilized on the dextran surface of the SPR crystal. Through a microflow system, a solution with the prey analyte is injected over the bait layer. As the prey analyte binds the bait ligand, an increase in SPR signal (expressed in response units, RU) is observed. After desired association time, a solution without the prey analyte (usually the buffer) is injected on the microfluidics that dissociates the bound complex between bait ligand and prey analyte. Now as the prey analyte dissociates from the bait ligand, a decrease in SPR signal (expressed in resonance units, RU) is observed. From these association ('on rate', ka) and dissociation rates ('off rate', kd), the equilibrium dissociation constant ('binding constant', KD) can be calculated.
The actual SPR signal can be explained by the electromagnetic 'coupling' of the incident light with the surface plasmon of the gold layer. This plasmon can be influenced by the layer just a few nanometer across the gold–solution interface i.e. the bait protein and possibly the prey protein. Binding makes the reflection angle change;

Thermodynamic analysis
As SPR biosensors facilitate measurements at different temperatures, thermodynamic analysis can be performed to obtain a better understanding of the studied interaction. By performing measurements at different temperatures, typically between 4 and 40 °C, it is possible to relate association and dissociation rate constants with activation energy and thereby obtain thermodynamic parameters including binding enthalpy, binding entropy, Gibbs free energy and heat capacity.Pair-wise epitope mapping
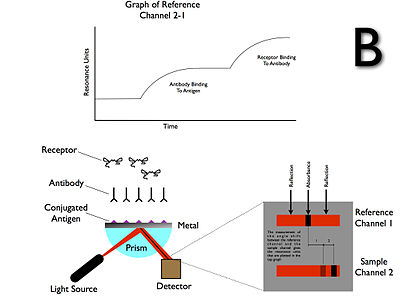
As SPR allows real-time monitoring, individual steps in sequential binding events can be thoroughly assessed when investigating the suitability between antibodies in a sandwich configuration. Additionally, it allows the mapping of epitopes as antibodies of overlapping epitopes will be associated with an attenuated signal compared to those capable of interacting simultaneously.Magnetic plasmon resonance
Recently, there has been an interest in magnetic surface plasmons. These require materials with large negative magnetic permeability, a property that has only recently been made available with the construction of metamaterials.Waves in plasmas

In plasma physics, waves in plasmas are an interconnected set of particles and fields which propagate in a periodically repeating fashion. A plasma is a quasineutral, electrically conductive fluid. In the simplest case, it is composed of electrons and a single species of positive ions, but it may also contain multiple ion species including negative ions as well as neutral particles. Due to its electrical conductivity, a plasma couples to electric and magnetic fields. This complex of particles and fields supports a wide variety of wave phenomena.
Terminology and classification
 , implying that an electrostatic wave must be purely longitudinal. An electromagnetic wave, in contrast, must have a transverse component, but may also be partially longitudinal.
, implying that an electrostatic wave must be purely longitudinal. An electromagnetic wave, in contrast, must have a transverse component, but may also be partially longitudinal.
Waves can be further classified by the oscillating species. In most plasmas of interest, the electron temperature is comparable to or larger than the ion temperature. This fact, coupled with the much smaller mass of the electron, implies that the electrons move much faster than the ions. An electron mode depends on the mass of the electrons, but the ions may be assumed to be infinitely massive, i.e. stationary. An ion mode depends on the ion mass, but the electrons are assumed to be massless and to redistribute themselves instantaneously according to the Boltzmann relation. Only rarely, e.g. in the lower hybrid oscillation, will a mode depend on both the electron and the ion mass.
The various modes can also be classified according to whether they propagate in an unmagnetized plasma or parallel, perpendicular, or oblique to the stationary magnetic field. Finally, for perpendicular electromagnetic electron waves, the perturbed electric field can be parallel or perpendicular to the stationary magnetic field.
| EM character | oscillating species | conditions | dispersion relation | name |
|---|---|---|---|---|
| electrostatic | electrons |  |
 |
plasma oscillation (or Langmuir wave) |
 |
 |
upper hybrid oscillation | ||
| ions |  |
 |
ion acoustic wave | |
 (nearly) (nearly) |
 |
electrostatic ion cyclotron wave | ||
 (exactly) (exactly) |
![\omega ^{2}=[(\Omega _{c}\omega _{c})^{{-1}}+\omega _{i}^{{-2}}]^{{-1}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/3a5987e075bb26f78b85bd0a847bba891a30ff44) |
lower hybrid oscillation | ||
| electromagnetic | electrons |  |
 |
light wave |
 |
 |
O wave | ||
 |
 |
X wave | ||
 (right circ. pol.) (right circ. pol.) |
 |
R wave (whistler mode) | ||
 (left circ. pol.) (left circ. pol.) |
 |
L wave | ||
| ions |  |
none | ||
 |
 |
Alfvén wave | ||
 |
 |
magnetosonic wave |
 - wave frequency,
- wave frequency,
 - wave number,
- wave number,
 - speed of light,
- speed of light,
 - plasma frequency,
- plasma frequency,
 - ion plasma frequency,
- ion plasma frequency,
 - electron gyrofrequency,
- electron gyrofrequency,
 - proton gyrofrequency,
- proton gyrofrequency,
 - upper hybrid frequency,
- upper hybrid frequency,
 - plasma "sound" speed,
- plasma "sound" speed,
 - plasma Alfvén speed
- plasma Alfvén speed
Sensors
There are two main sensors for Waves, and these field signals to the frequency receivers.Both sensors are attached to main spacecraft body.- Dipole antenna
- Magnetic search coil
Frequency receiver
There are two frequency receivers that each cover certain bands, a high band and a low band, which in turn has different receiving sections. The receivers are housed in the Juno Radiation Vault along with other electronics.Breakdown:
- High Frequency Receiver
- High Frequency Receiver ~100 kHz - 40 MHz (Spectrum (High) and Waveform (Low))
- High Frequency Waveform Receiver
- Baseband receiver includes:
- variable-gain amplifier
- 100 Hz to 3 MHz band-pass filter
- 12-bit analog-to-digital converter
- Double sideband heterodyne receiver for 3 to 40 MHz (swept frequency receiver)[6]
- Baseband receiver includes:
- Low Frequency Receiver
- High, Low Frequency Receiver ~10 kHz - 150 kHz (E waveform)
- Low, Low Frequency Receiver ~50 Hz - 20 kHz (E and B waveforms)
Data Processing Unit (DPU)
- Y180 intellectual property core
- Floating point arithmetic unit
Multimedia
Waves has detected radio emissions from the Jupiter auroras, the most powerful known in the Solar System to date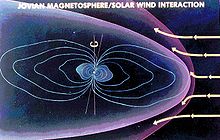 This illustration shows how the Jovian magnetosphere is thought to interact with the incoming solar wind (yellow arrows)
This illustration shows how the Jovian magnetosphere is thought to interact with the incoming solar wind (yellow arrows) 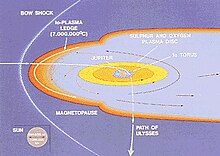 The path of the Ulysses spacecraft through the magnetosphere of Jupiter in 1992, shows the location of the Jovian bow shock.
The path of the Ulysses spacecraft through the magnetosphere of Jupiter in 1992, shows the location of the Jovian bow shock.
Jupiter aurora; the bright spot at far left is the end of field line to Io; spots at bottom lead to Ganymede and Europa.
Captured by Hubble Space Telescope from Earth orbit in ultraviolet,
represented one way to study Jupiter's aurora, which will also be
studied by the Waves instrument from orbit, detecting radio and plasma
waves in situ

Waves being installed on Juno spacecraft

cyborgs, cloning and the science of new life
Sci-fi gone real is a series that focusses on the extravagant concepts dreamt up in the world of sci-fi, exploring just how close they are to becoming a reality.In this piece we discuss ‘new life’. From artificial intelligence to terraforming, mad scientists all over the world are hard at work dissecting and, potentially, changing the very fabric of existence.
Cloning
Cloning is a concept that has been explored so extensively in sci-fi works that it almost seems a reality. But, like with most of the other entries on this list, it looks like truth is becoming stranger than fiction, and has been for longer than you might think.Cyborgs
With recent advances in robotics and biomechatronics, the reality of a cyborg is no longer on the horizon; it’s here, and has been for quite some time. Currently, there are a plethora of companies that offer the cutting edge in advanced prosthetics, not only to rectify physiological impairments but to enhance our human abilities altogether.For amputees and people who were born without certain limbs, these advanced prosthetics provide them with the opportunity to live their lives as most of us do, freeing them from the obstacles and hassles that these impairments cause.
On the other side of the spectrum, we have fully functional human beings that are looking at biomechatronics as a means of further enhancing the human condition.

Artificial intelligence
the notion of artificial intelligence (AI) to quite some extent. But in the last few years, in reality, we have made giant leaps in the realisation of an intelligent artificial consciousness.
At this point in time, there are already several operational AIs in existence that are only getting smarter by the day.Beyond these latest and greatest AI developments, there are also quite a few AIs a number of us interact with on a daily basis. Siri, Netflix, Amazon… all of these make use of machine-learning to better adapt to natural speech patterns, viewing habits, and buying habits, respectively.
Terraforming
Terraforming is the process of altering an uninhabitable planet’s atmosphere so that it is able to support human life. Now considering the extreme complexity and sheer magnitude of such a project, it might not come as a surprise that we are not anywhere near pulling off such an incredible feat.That doesn’t mean that scientists have not yet pondered its plausibility though. Just because we aren’t close, doesn’t mean we aren’t moving closer to realising this pipe dream.
Besides the abovementioned issues, one of the major problems with terraforming is that it’s not exactly a copy-and-paste endeavour. Each planet has an enormous amount of variables to consider (circumference, liquid water, soil, atmosphere density, etc.), with not a single planet sharing even the minutest of details.
Mars has long been considered the best candidate for a new human home as it is widely believed by many scientist to have once boasted an atmosphere very similar to Earth’s.
+++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++
e- Cyborg and Cloning


e- Cyborg and Cloning
+++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++










Tidak ada komentar:
Posting Komentar